Acids And Bases Worksheet Answer Key
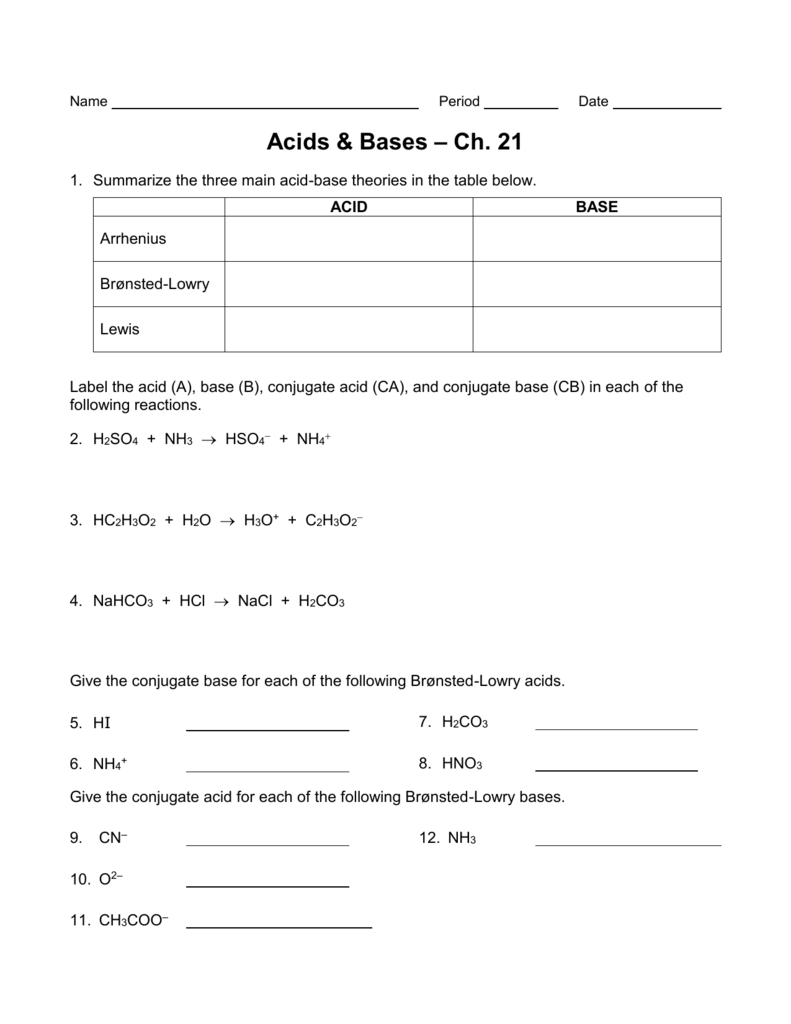
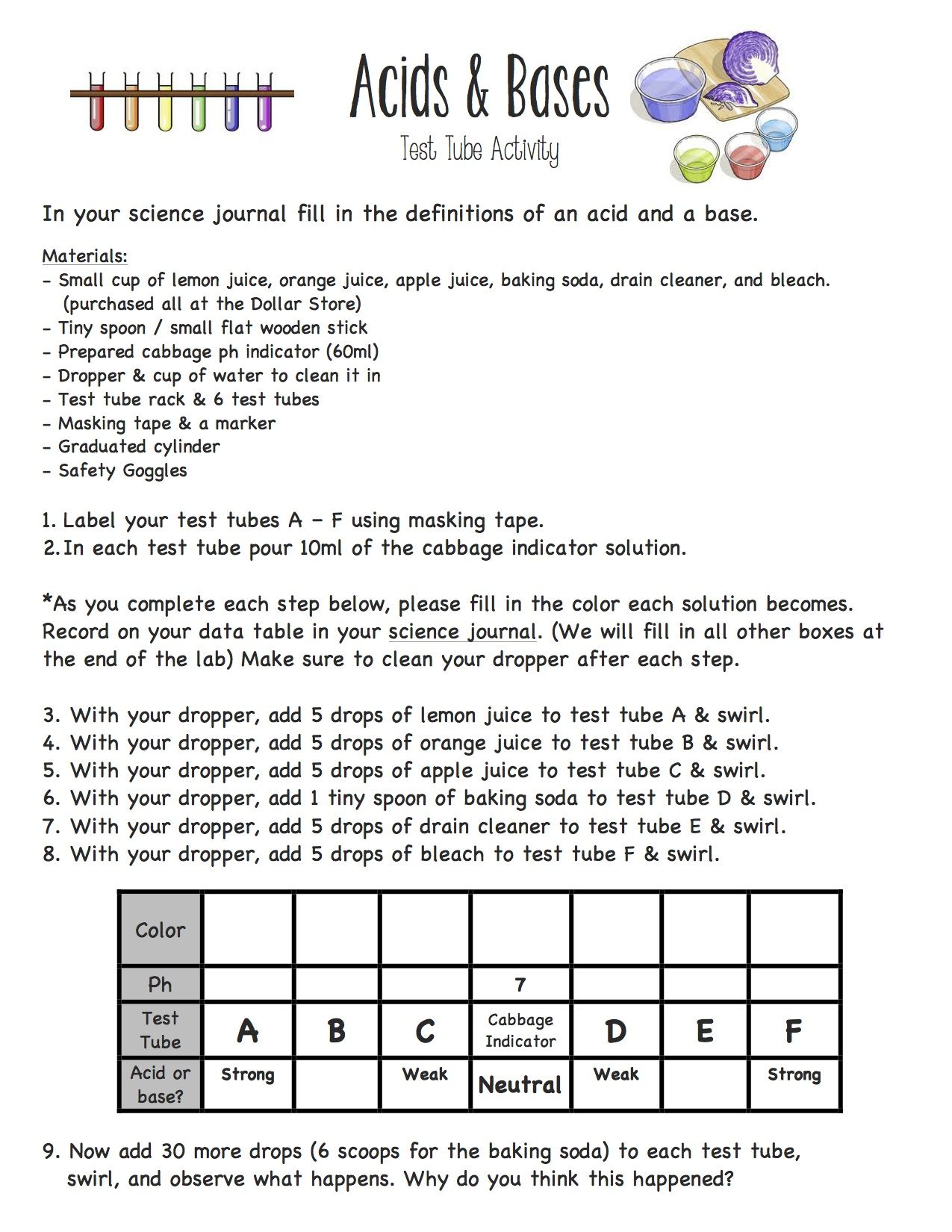
Acids And Bases Worksheet Answer Key - Web determine the ka for a 0 solution of a monoprotic acid. This is the dissociation of a weak monoprotic acid: Web these acids and bases worksheets cover the following topics: Some of the worksheets for this concept are acids bases salts work answer key, chapter 19 acids bases salts work answers, acids bases and salts answers key, chapter 19 1 acids bases and salts work answers, chapter 19 1 acids bases and salts work answers, chapter 19. Why is an aqueous solution of nanh2. Characteristics of acids important to know. O] = [a] = 2. So set up an ice. General word equations for the reactions of an acid with a metal, metal oxide, metal hydroxide and a metal carbonate; A solution of poh 6.0 or one of poh 8.0. Web acids versus bases 1. The source of both hco − 3 and co2 − 3 is h2co3. Which compounds from the list are acidic? All ph values that follow are measurements of 0.010 m solutions. They include questions on identifying acids and bases, naming and writing formulas for salts, predicting products of the three major types of neutralization reactions. Web explain your answer. All ph values that follow are measurements of 0.010 m solutions. Or 1% of 0 = 2. Which compounds from the list are acidic? 5th grade science worksheets and answer key, study guides. Or 1% of 0 = 2. Web explain your answer. Web a) what is the ratio of weak acid molecules to water molecules in a 0.10 m solution? Which compounds from the list are basic? Name the following binary acids: Or 1% of 0 = 2. * ph and poh calculations pdf. This is the dissociation of a weak monoprotic acid: Why is an aqueous solution of nanh2. Hcl hydrochloric acid hf hydrofluoric acid h 2se hydroselenic acid hi hydroiodic acid h 2s hydrosulfuric acid hbr hydrobromic acid Compare and contrast acids and bases by completing the following table. Common properties of salts, such as sodium chloride (nacl). Web a) what is the ratio of weak acid molecules to water molecules in a 0.10 m solution? Hcl hydrochloric acid hf hydrofluoric acid h 2se hydroselenic acid hi hydroiodic acid h 2s hydrosulfuric acid hbr hydrobromic acid Classify each. The ph of acidic and alkaline solutions; Null 𝑂 %] using the percent dissociation the 1% dissociation means. Why are aqueous solutions of salts such as cacl2 neutral? They include questions on identifying acids and bases, naming and writing formulas for salts, predicting products of the three major types of neutralization reactions and balancing equations. Web acids, bases and salts. * dissociation & ionization pdf. Web acids and bases worksheet 1 grade/level: (a) + h 3po 4 (b) nh 4oh (c) mg(oh) 2 (d) has a ph of 4 (e) has a ph of 9 ( f ) sulphurous acid (g) hydrogen bromide (h) potassium hydroxide Some of the worksheets for this concept are acids bases salts work answer key,. Which compounds from the list are basic? Null 𝑂 %] using the percent dissociation the 1% dissociation means. Alkalinity = [hco − 3] + 2[co2 − 3] + [oh −] − [h +]. A solution of poh 6.0 or one of poh 8.0. 5th grade science worksheets and answer key, study guides. The solution has a 1% dissociation. Which compounds from the list are acidic? This is the dissociation of a weak monoprotic acid: Web acids, bases and salts worksheets contains 15 student worksheets with over 150 questions. Web a 0.1 m solution of an acid with \(k_a = 1 \times 10^{‐4}\) or one with \(k_a = 4 \times 10^{‐5}\) a 0.1. The solution has a 1% dissociation. Web acids and bases worksheet 1 grade/level: This is the dissociation of a weak monoprotic acid: General word equations for the reactions of an acid with a metal, metal oxide, metal hydroxide and a metal carbonate; Web a) what is the ratio of weak acid molecules to water molecules in a 0.10 m solution? Acid base acid base (a) hclo4(aq) k2co3(aq) (b) hbr(aq) nacn(aq) 18. By reversing the reaction in which a substance acts as a proton donor, we see that the product is itself a proton acceptor. General word equations for the reactions of an acid with a metal, metal oxide, metal hydroxide and a metal carbonate; Explain why the basicity of soil is defined in this way. Some of the worksheets for this concept are acids bases salts work answer key, chapter 19 acids bases salts work answers, acids bases and salts answers key, chapter 19 1 acids bases and salts work answers, chapter 19 1 acids bases and salts work answers, chapter 19. Hcl hydrochloric acid hf hydrofluoric acid h 2se hydroselenic acid hi hydroiodic acid h 2s hydrosulfuric acid hbr hydrobromic acid Classify each of the following as an acid or a base. A solution of poh 6.0 or one of poh 8.0. Which compounds from the list are basic? They include questions on identifying acids and bases, naming and writing formulas for salts, predicting products of the three major types of neutralization reactions and balancing equations. 7th grade science worksheets and answer key, study guides. Web acids, bases and salts. Web a) what is the ratio of weak acid molecules to water molecules in a 0.10 m solution? Which compounds from the list are acidic? Or 1% of 0 = 2. Web determine the ka for a 0 solution of a monoprotic acid. This is the dissociation of a weak monoprotic acid: Common properties of salts, such as sodium chloride (nacl). Which compounds from the list are n? Web acids, bases and salts worksheets contains 15 student worksheets with over 150 questions. Explain why the basicity of soil is defined in this way. Why is an aqueous solution of nanh2. Web acids versus bases 1. A solution of poh 6.0 or one of poh 8.0. 5th grade science worksheets and answer key, study guides. Which compounds from the list are basic? Why are aqueous solutions of salts such as cacl2 neutral? Alkalinity = [hco − 3] + 2[co2 − 3] + [oh −] − [h +]. Web explain your answer. Some of the worksheets for this concept are acids bases salts work answer key, chapter 19 acids bases salts work answers, acids bases and salts answers key, chapter 19 1 acids bases and salts work answers, chapter 19 1 acids bases and salts work answers, chapter 19. This is the dissociation of a weak monoprotic acid: Which compounds from the list are n? Classify each of the following as an acid or a base. (a) + h 3po 4 (b) nh 4oh (c) mg(oh) 2 (d) has a ph of 4 (e) has a ph of 9 ( f ) sulphurous acid (g) hydrogen bromide (h) potassium hydroxide They include questions on identifying acids and bases, naming and writing formulas for salts, predicting products of the three major types of neutralization reactions and balancing equations. The ph of acidic and alkaline solutions;Acid And Base Worksheet
Acid And Bases Worksheet Answers
13 3D Shape Matching Worksheet /
Acids and Bases Worksheet Answers
Acid And Base Worksheet
Introduction To Acids And Bases Worksheet Answers
Naming Acids Worksheet Answers Gbgyaba Practice Test Answer Key Free
Acids And Bases Worksheet
Ph Worksheet Answer Key
Acids & Bases And Ph Worksheet Pdf Answer Key Islero Guide Answer for
7Th Grade Science Worksheets And Answer Key, Study Guides.
Web Acids, Bases And Salts.
General Word Equations For The Reactions Of An Acid With A Metal, Metal Oxide, Metal Hydroxide And A Metal Carbonate;
So Set Up An Ice.
Related Post: