Acids And Bases Worksheet Answers
Acids And Bases Worksheet Answers - Acids in order of increasing strength: Find [oh] and ph for the following bases in solution: Web which of them do not need to be an aqueous solution? An acid is a type of sour substance. Browse acids and bases questions or use our advanced search to find existing questions while filtering by. The concentration of hydrogen ion in. Web use our test maker™ to create your own printable that fits your needs. Given the same initial concentration of each acid, the highest. What are acids and bases? What is the ph of the solution? Browse acids and bases questions or use our advanced search to find existing questions while filtering by. Web a 0.1 m solution of an acid with \(k_a = 1 \times 10^{‐4}\) or one with \(k_a = 4 \times 10^{‐5}\) a 0.1 m solution of an acid with \(pk_a = 1.0\) or one with \(pk_a =. 0 koh koh is a. What are acids and bases? Do the questions, and follow along. Browse acids and bases questions or use our advanced search to find existing questions while filtering by. Find [oh] and ph for the following bases in solution: H2o 2.4 25 cm3 water is added tot 75 cm3 of a 0,13 mol·dm−3. H2o 2.4 25 cm3 water is added tot 75 cm3 of a 0,13 mol·dm−3. Browse acids and bases questions or use our advanced search to find existing questions while filtering by. Acids and bases are substances we encounter daily. An arrhenius acid produces h+ and an. Some of the worksheets for this concept are chem1612 work 6 acids and bases. 0 koh koh is a strong base so it dissociates completely. The concentration of hydrogen ion in. Web the worksheet requires each student to balance, identify the formula and phase of the salt formed, and name the acid, base, and salt in 18 neutralization reactions. Acids in order of increasing strength: A base is a type of bitter substance. + h jo 'z ppon u! Do the questions, and follow along. A base is a type of bitter substance. Web a) what is the ratio of weak acid molecules to water molecules in a 0.10 m solution? Web which of them do not need to be an aqueous solution? Is the solution acidic or basic? Web the worksheet requires each student to balance, identify the formula and phase of the salt formed, and name the acid, base, and salt in 18 neutralization reactions. 0 koh koh is a strong base so it dissociates completely. Web use our test maker™ to create your own printable that fits your needs. Describe. Examples of acids are lemon juice and vinegar. Vinegar, bleach, baking soda, and cola. Acids in order of increasing strength: The concentration of hydrogen ion in. What is the ph of the solution? An acid is a type of sour substance. Web a 0.1 m solution of an acid with \(k_a = 1 \times 10^{‐4}\) or one with \(k_a = 4 \times 10^{‐5}\) a 0.1 m solution of an acid with \(pk_a = 1.0\) or one with \(pk_a =. Given the same initial concentration of each acid, the highest. In the following reaction. Web which of them do not need to be an aqueous solution? Some of the worksheets for this concept are chem1612 work 6 acids and bases model 1 strong and, chm 130 acids. An arrhenius acid produces h+ and an. Do the questions, and follow along. Describe and name acids and bases. Web use our test maker™ to create your own printable that fits your needs. Web 100tps save asvq (110! Aseq—ppe.asvq acp sassvd pup uoz v dil saaz; Vinegar, bleach, baking soda, and cola. An acid is a type of sour substance. Examples of acids are lemon juice and vinegar. What is the hydroxide ionconcentration? Web a) what is the ratio of weak acid molecules to water molecules in a 0.10 m solution? ) chooh (aq) ) h2s (aq) h2o (l) nh3. Web 100tps save asvq (110! 0 koh koh is a strong base so it dissociates completely. A base is a type of bitter substance. H2o 2.4 25 cm3 water is added tot 75 cm3 of a 0,13 mol·dm−3. H2o 2.3 write down the conjugated acids of the following bases: In the following reaction in. + h jo 'z ppon u! Find [oh] and ph for the following bases in solution: Web a 0.1 m solution of an acid with \(k_a = 1 \times 10^{‐4}\) or one with \(k_a = 4 \times 10^{‐5}\) a 0.1 m solution of an acid with \(pk_a = 1.0\) or one with \(pk_a =. Vinegar, bleach, baking soda, and cola. Given the same initial concentration of each acid, the highest. Aseq—ppe.asvq acp sassvd pup uoz v dil saaz; Web which of them do not need to be an aqueous solution? What are acids and bases? What is the ph of the solution? An acid is a type of sour substance. A base is a type of bitter substance. H2o 2.4 25 cm3 water is added tot 75 cm3 of a 0,13 mol·dm−3. What is the ph of the solution? Web a 0.1 m solution of an acid with \(k_a = 1 \times 10^{‐4}\) or one with \(k_a = 4 \times 10^{‐5}\) a 0.1 m solution of an acid with \(pk_a = 1.0\) or one with \(pk_a =. Browse acids and bases questions or use our advanced search to find existing questions while filtering by. 0 koh koh is a strong base so it dissociates completely. Web which of them do not need to be an aqueous solution? Web a) what is the ratio of weak acid molecules to water molecules in a 0.10 m solution? H2o 2.3 write down the conjugated acids of the following bases: Is the solution acidic or basic? Acids and bases are substances we encounter daily. In the following reaction in. Given the same initial concentration of each acid, the highest. Some of the worksheets for this concept are chem1612 work 6 acids and bases model 1 strong and, chm 130 acids. Describe and name acids and bases. Acids in order of increasing strength:12 Best Images of Acid Rain And Ph Worksheet Answers AcidBase
Acid And Base Worksheet
Overview Acids Bases And Salts Worksheet Answers
Acid/Base Ms Beaucage
Worksheet naming acids and bases
Introduction To Acids And Bases Worksheet Answers
Acid And Base Worksheet
Acids And Bases Worksheet Answers
Solutions Acids and Bases Worksheet New Acids and Bases Lesson 1
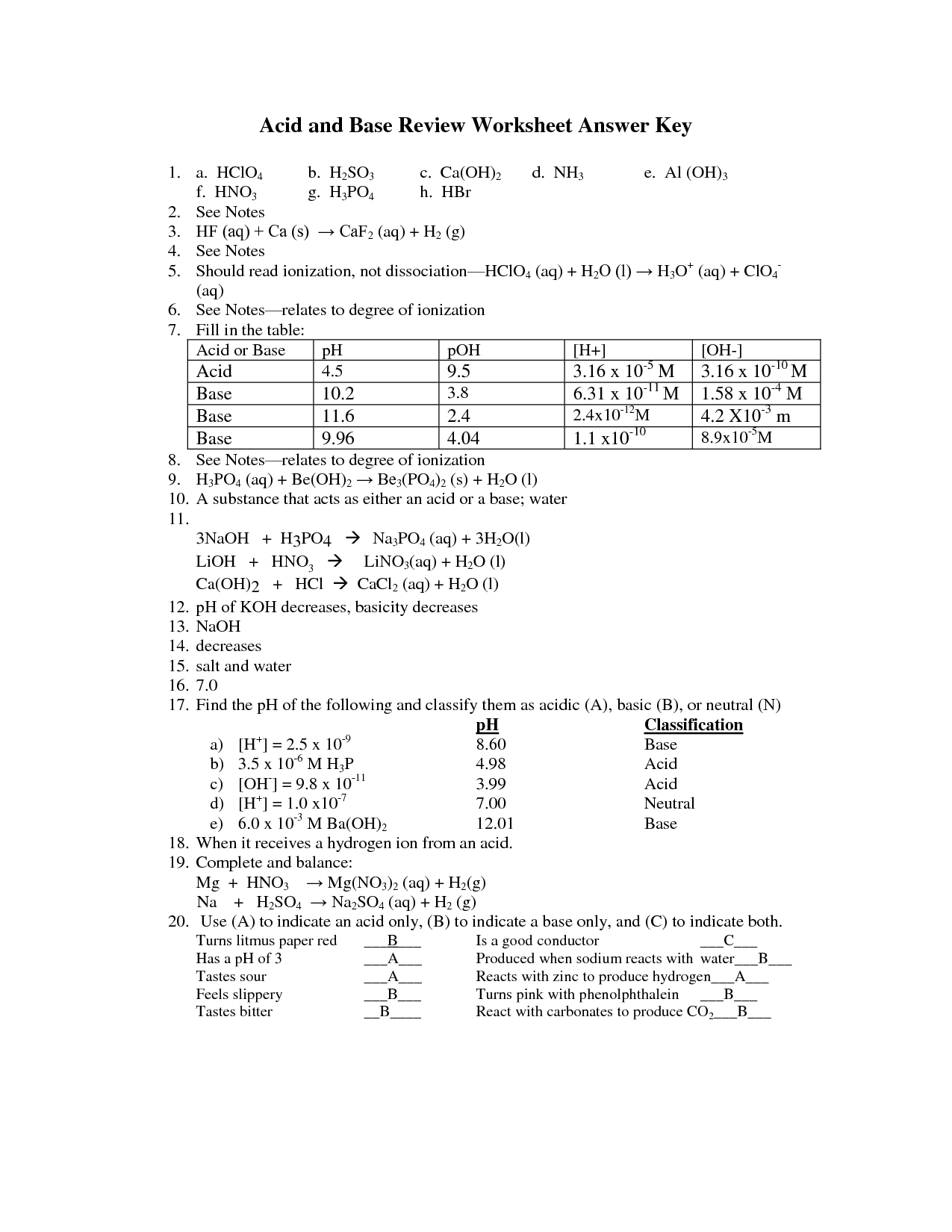
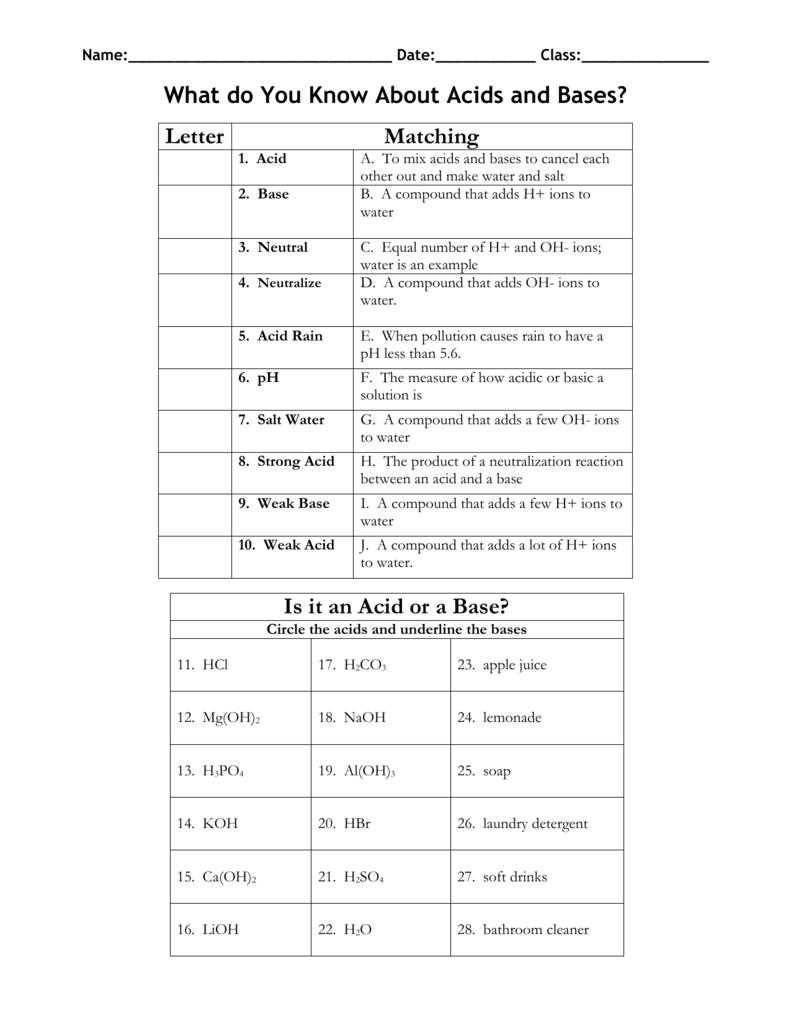
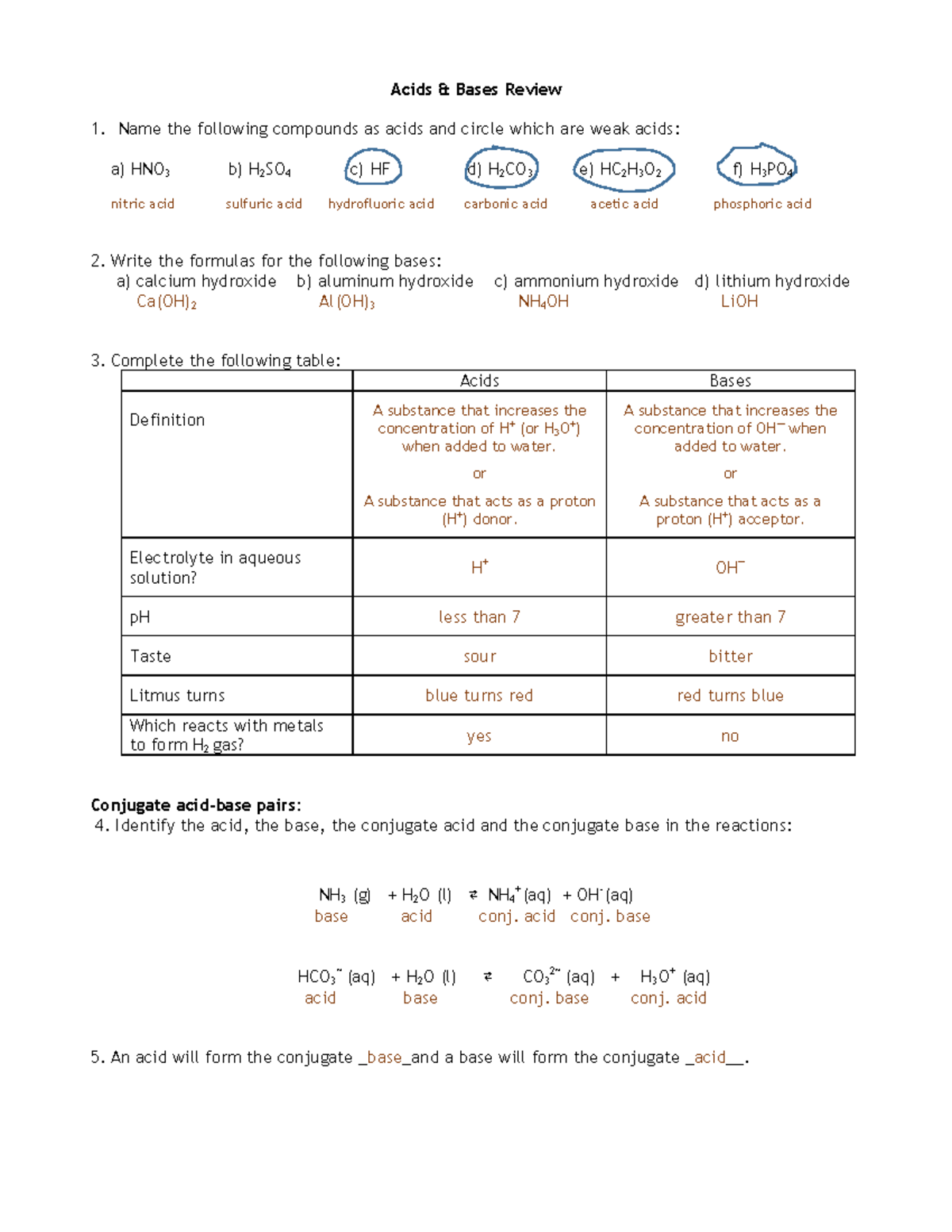
Acids and Bases Review Worksheet Answers Acids & Bases Review 1. Name
An Arrhenius Acid Produces H+ And An.
Web 100Tps Save Asvq (110!
Aseq—Ppe.asvq Acp Sassvd Pup Uoz V Dil Saaz;
What Are Acids And Bases?
Related Post: