Acids Bases & Ph Worksheet
Acids Bases & Ph Worksheet - Neutral solutions have a ph of 7. The ph of the solution is 2. Name of your substance : Web learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Calculate the ph of a solution with [h +] = 1 m. So set up an ice table: This is the dissociation of a weak monoprotic acid: Web which solution has a higher ph? Assess your students on all aspects of acids, bases, and the ph scale with this great, detailed quiz! Determine the ka for a 0 solution of nitrous acid (hno 2). Write a chemical equation, identify the limiting reactant (if there is one), and. These worksheets provide a comprehensive. Assess your students on all aspects of acids, bases, and the ph scale with this great, detailed quiz! Determine the ka for a 0 solution of nitrous acid (hno 2). Calculate the ph of a 0.01 m solution of hcl. Calculate the ph of a solution with [h +] = 1 m. The ph of the solution is 2. Calculate the ph of a. Acids are characteristically sour, corrosive, and neutralized by bases. Web learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Calculate the ph of a. Calculate the ph of a 0.01 m solution of hcl. These worksheets provide a comprehensive. This is the dissociation of a weak monoprotic acid: Perfect for any chemistry or physical science. (1) identifying ranges of the ph scale, (2) differentiating between acidic and basic solutions, (3) caluclation of ph logarithms, and. A 0.1 m solution of an acid with \(k_a = 1 \times 10^{‐4}\) or one with \(k_a = 4 \times 10^{‐5}\) a 0.1 m solution of an acid with. Web learn for free about math, art, computer programming, economics, physics,. Web which solution has a higher ph? Web acids and bases worksheets are an essential tool for teachers looking to engage their students in the exciting world of science. Remember to add the acid to the water and not the water to the acid! Calculate the ph of a 0.01 m solution of hcl. Grade 7 acids and bases wordsearch. Calculate the ph of a. Grade 7 acids and bases wordsearch. The ph of the solution is 2. Worksheets are acid and base ph calculations supplemental work key, work 1 acids bases indicators, strong. Web acids have a ph below 7 and bases have a ph above 7. Perfect for any chemistry or physical science. (1) identifying ranges of the ph scale, (2) differentiating between acidic and basic solutions, (3) caluclation of ph logarithms, and. Calculate the ph of a. The ph of the solution is 2. These worksheets provide a comprehensive. Calculate the ph of a. Worksheets are acid and base ph calculations supplemental work key, work 1 acids bases indicators, strong. Neutral solutions have a ph of 7. Web acids and bases are chemical substances that react with each other and may affect ph levels. Web the ph worksheet includes the following: Assess your students on all aspects of acids, bases, and the ph scale with this great, detailed quiz! Web acids, bases, and ph worksheet experiment 1 substance cabbage indicator color approximate ph definitions experiment 2 pick one of the substances above and add it to water. Calculate the ph of a. Acids are characteristically sour, corrosive, and neutralized by bases.. Neutral solutions have a ph of 7. A 0.1 m solution of an acid with \(k_a = 1 \times 10^{‐4}\) or one with \(k_a = 4 \times 10^{‐5}\) a 0.1 m solution of an acid with. Web acids and bases worksheet 2. Determine the equilibrium concentration for [𝑯. Calculate the ph of a 0.01 m solution of hcl. Web acids have a ph below 7 and bases have a ph above 7. Web acids and bases are chemical substances that react with each other and may affect ph levels. Calculate the ph of a solution with [h +] = 1 m. So set up an ice table: Web which solution has a higher ph? Acids are characteristically sour, corrosive, and neutralized by bases. Calculate the ph of a 0.01 m solution of hcl. Determine the ka for a 0 solution of nitrous acid (hno 2). This is the dissociation of a weak monoprotic acid: The ph of the solution is 2. A 0.1 m solution of an acid with \(k_a = 1 \times 10^{‐4}\) or one with \(k_a = 4 \times 10^{‐5}\) a 0.1 m solution of an acid with. Perfect for any chemistry or physical science. Determine the equilibrium concentration for [𝑯. Web learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. (1) identifying ranges of the ph scale, (2) differentiating between acidic and basic solutions, (3) caluclation of ph logarithms, and. Remember to add the acid to the water and not the water to the acid! Grade 7 acids and bases wordsearch. Web acids, bases, and ph worksheet experiment 1 substance cabbage indicator color approximate ph definitions experiment 2 pick one of the substances above and add it to water. Write a chemical equation, identify the limiting reactant (if there is one), and. Web acids and bases worksheet 2. Web acids and bases worksheets are an essential tool for teachers looking to engage their students in the exciting world of science. Perfect for any chemistry or physical science. Name of your substance : Web acids have a ph below 7 and bases have a ph above 7. Determine the equilibrium concentration for [𝑯. Assess your students on all aspects of acids, bases, and the ph scale with this great, detailed quiz! Acids are characteristically sour, corrosive, and neutralized by bases. Neutral solutions have a ph of 7. These worksheets provide a comprehensive. The ph of the solution is 2. This is the dissociation of a weak monoprotic acid: Web the ph worksheet includes the following: A 0.1 m solution of an acid with \(k_a = 1 \times 10^{‐4}\) or one with \(k_a = 4 \times 10^{‐5}\) a 0.1 m solution of an acid with. Write a chemical equation, identify the limiting reactant (if there is one), and. Web which solution has a higher ph? (1) identifying ranges of the ph scale, (2) differentiating between acidic and basic solutions, (3) caluclation of ph logarithms, and.the ph scale worksheet answers
Acids And Bases Ph Scale Worksheet Acids, Bases, and the pH Scale
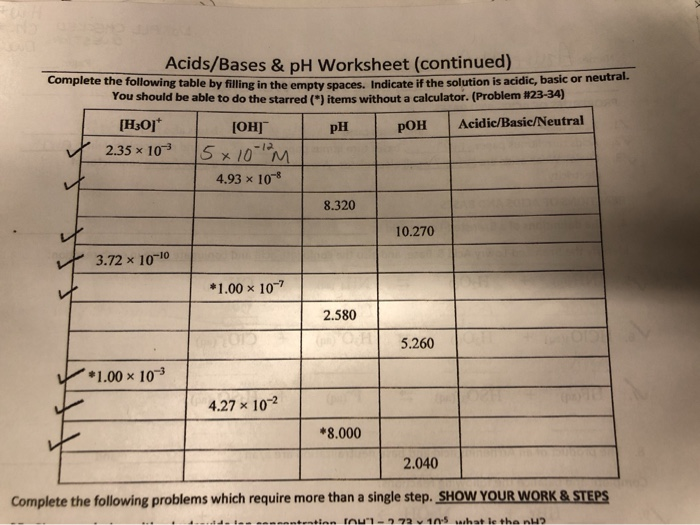
Solved Can you explain how to do at least one row and than I
Acids & Bases And Ph Worksheet Pdf Answer Key Islero Guide Answer for
Blank Ph Scale Worksheet Beautiful Acids and Bases Lessons Tes Teach
Acids And Bases Ph Scale Worksheet Acids, Bases, and the pH Scale
Acids And Bases Worksheet Physical Science Worksheet AcidBase
Acids And Bases Worksheet Ph Acids And Bases Interactive Worksheet
Acids and Bases Coloring Worksheet top Acids & Bases Ph Scale Science
Ph Acids and Bases Worksheet 2022 2023 EduVark
Calculate The Ph Of A.
Web Acids And Bases Are Chemical Substances That React With Each Other And May Affect Ph Levels.
Worksheets Are Acid And Base Ph Calculations Supplemental Work Key, Work 1 Acids Bases Indicators, Strong.
Web Learn For Free About Math, Art, Computer Programming, Economics, Physics, Chemistry, Biology, Medicine, Finance, History, And More.
Related Post: