Answer Key Stoichiometry Practice Worksheet Answers
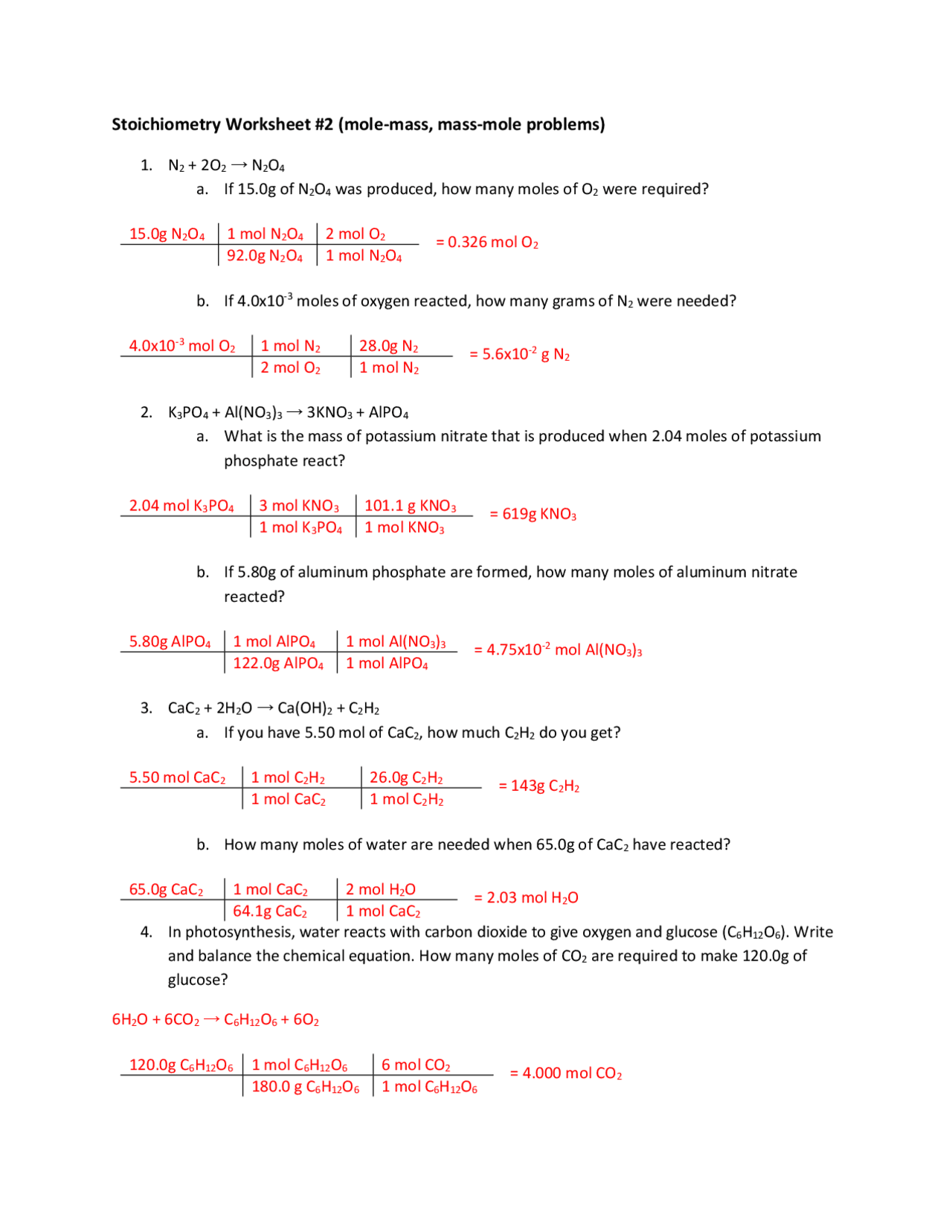
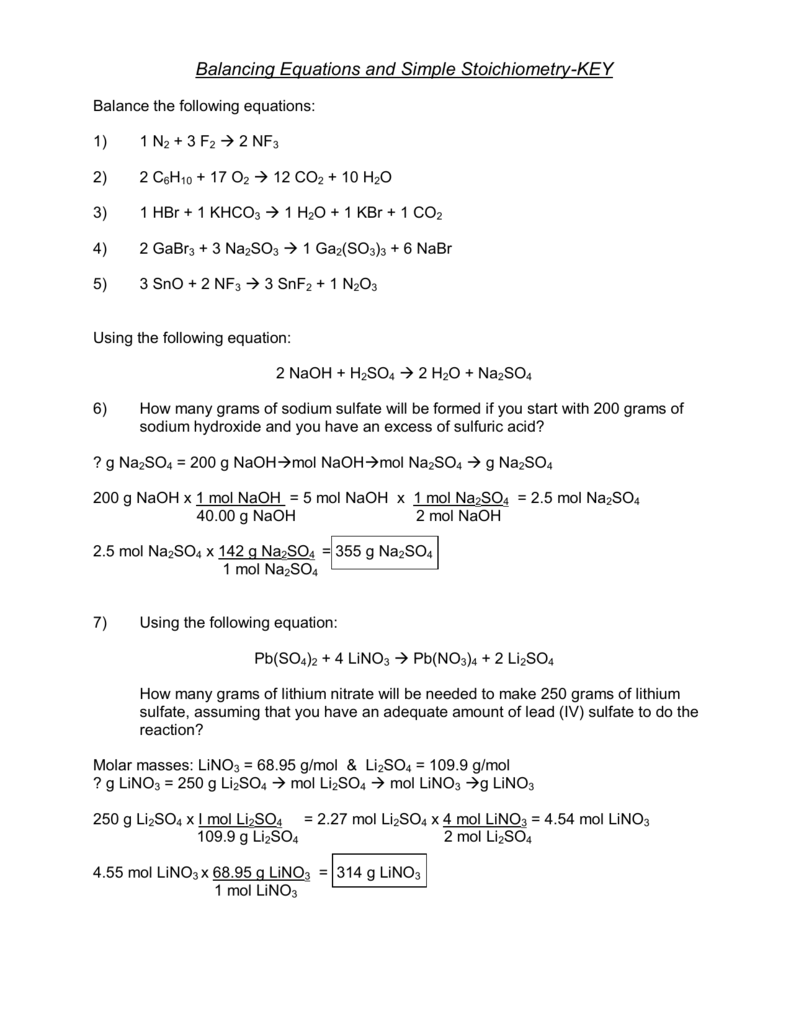
Answer Key Stoichiometry Practice Worksheet Answers - How many moles of h2 would be required to produce 5.0 moles of water? Web stoichiometry calculation practice worksheet. (choice a) 88 88 8 8 88 grams. How many moles of o 2 can be produced by. The links to the corresponding topics are given below. What mass of h2o is formed when h2 reacts with 384 g of o2? H2so4 + 2naoh ( na2so4 + 2h2o. O 2 / h 2o d. Stoichiometry (answer key) balance the following chemical reactions: 3)1 hbr + 1 khco3 ( 1 h2o + 1 kbr + 1 co2. C 4h 10 / h 2o 2. 2h2 + o2 ( 2h2o. (c) how many grams of butane burned? C 6 h 12 o 6 (s) 2 c 2 h 5 oh (l). Limiting reactant example problem 1 edited. Each worksheet is clearly labeled for each lesson and is fully adaptable to any chemistry classroom. (d) how much oxygen was used up in. 4)2 gabr3 + 3 na2so3 ( 1 ga2(so3)3 + 6 nabr. 3)1 hbr + 1 khco3 ( 1 h2o + 1 kbr + 1 co2. 1) ___ n2 + ___ f2 ___ nf3 2) ___ c6h10. Test prep > mcat > foundation 4: C 6 h 12 o 6 (s) 2 c 2 h 5 oh (l). 3)1 hbr + 1 khco3 ( 1 h2o + 1 kbr + 1 co2. Noretain the present instance of the string and advance to the next instance. 2 co + o 2 2 co 2 b. (c) how many grams of butane burned? H2so4 + 2naoh ( na2so4 + 2h2o. Web solve the following solutions stoichiometry problems: This is a bundle of homework worksheets that i use with my classes when i teach stoichiometry. 1)1 n2 + 3 f2 ( 2 nf3. How many moles of o 2 can be produced by. (c) how many grams of butane burned? C 4h 10 / o 2 b. The topics for each worksheet is as follows: Write the balanced chemical equations of each reaction: Fermentation is a complex chemical process of making wine by converting glucose into ethanol and carbon dioxide: 88 88 8 8 88 grams (choice b) 0 0 0 0 grams. 2)2 c6h10 + 17 o2 ( 12 co2 + 10 h2o. Web stoichiometry worksheet #1 answers 1. Given the equation 3a + b → c + d, you react 1. O 2 / co 2 c. Stoichiometry (answer key) balance the following chemical reactions: Is the limiting reactant because you have fewer moles of b than a. 40 40 4 0 40 grams (choice d. Mass to mass worksheet 2.0 ”. The links to the corresponding topics are given below. (b) how many moles of butane burned? C 4h 10 / o 2 b. 2 kno 3 2 kno 2 + o 2 c. Is the limiting reactant because you have fewer moles of b than a. C 6 h 12 o 6 (s) 2 c 2 h 5 oh (l). How many moles of o 2 can be produced by. The chemistry teacher on youtube. 1)1 n2 + 3 f2 ( 2 nf3. Cr(oh) 3 + 3 hclo 4 cr(clo 4) 3 + 3 h 2 o; What mass of h2o is formed when h2 reacts with 384 g of o2? 2h2 + o2 ( 2h2o. Web solve the following solutions stoichiometry problems: Web stoichiometry practice problems 1) lithium hydroxide reacts with hydrogen bromide to produce lithium bromide and water. The topics for each worksheet is as follows: Web stoichiometry practice problems 1) lithium hydroxide reacts with hydrogen bromide to produce lithium bromide and water. How many grams of silver chromate will precipitate when 150. All of the work is shown also. Calculate the mass of nh 3 that can be produced from the reaction of 125 g of ncl 3 according to the following equation: If you start with ten grams of lithium hydroxide, how many grams of lithium bromide will beproduced? Ml of 0.400 m potassium chromate? Great for extra practice worksheets! 2) ethylene (c2h4) reacts with oxygen gas to produce carbon dioxide and water. Web chm 130 stoichiometry worksheet the following flow chart may help you work stoichiometry problems. 2h2 + o2 ( 2h2o. The chemistry teacher on youtube. H 2 so 4 + 2 naoh na 2 so 4 + 2 h 2 o. C 4h 10 / co 2 e. 4 ch 3 nh 2 + 9 o 2 4 co 2 + 10 h 2 o + 2 n 2 f. Write the balanced chemical equations of each reaction: How many moles of o 2 can be produced by. (b) how many moles of butane burned? 3)1 hbr + 1 khco3 ( 1 h2o + 1 kbr + 1 co2. C 6 h 12 o 6 (s) 2 c 2 h 5 oh (l). 1) the combustion of a sample of butane, c4h10 (lighter fluid), produced 2.46 grams of water. (d) how much oxygen was used up in. Calculate the mass of nh 3 that can be produced from the reaction of 125 g of ncl 3 according to the following equation: Cr(oh) 3 + 3 hclo 4 cr(clo 4) 3 + 3 h 2 o; All of the work is shown also. 2 agno3(aq) + k2cro4(aq) ag2cro4(s) + 2 kno3(aq) = 12.4 g ag2cro4 = 13.3 g ag2cro4 2. Ml of 0.500 m silver nitrate are added to 100. 1) ___ n2 + ___ f2 ___ nf3 2) ___ c6h10 + ___ o2 ___ co2 + ___ h2o 3) ___ hbr + ___ khco3 ___ h2o + ___ kbr + ___ co2 4) ___ gabr3 + ___ na2so3 ___ ga2(so3)3 + ___ nabr 5) ___ sno + ___ nf3 ___ snf2 + ___ n2o3 2) ethylene (c2h4) reacts with oxygen gas to produce carbon dioxide and water. The topics for each worksheet is as follows: C 6 h 12 o 6 (s) 2 c 2 h 5 oh (l). 4)2 gabr3 + 3 na2so3 ( 1 ga2(so3)3 + 6 nabr. Web stoichiometry practice worksheet balancing equations and simple stoichiometry balance the following equations: Yesreplace the current instance of the string with the required replacement and advance to the subsequent instance. Great for extra practice worksheets! Is the limiting reactant because you have fewer moles of b than a. 1) the combustion of a sample of butane, c4h10 (lighter fluid), produced 2.46 grams of water.Ch. 45 Moles/Stoichiometry answers SHHSHonorsChemistry
Stoichiometry Worksheet With Answer Key
Stoichiometry Worksheet With Answer Key
Stoichiometry Worksheet Answer Key
Stoichiometry Worksheet 2 Answer Key
13 Best Images of Practice Balancing Equations Worksheet Key
Stoichiometry Worksheet Answer Key
10 Best Images of Stoichiometry Worksheet 2 Answer Key Chemistry
Stoichiometry Worksheet Answer Key
14 Stoichiometry Worksheet 2 Answer Key /
88 88 8 8 88 Grams (Choice B) 0 0 0 0 Grams.
The Chemistry Teacher On Youtube.
C 4H 10 / O 2 B.
(C) How Many Grams Of Butane Burned?
Related Post: