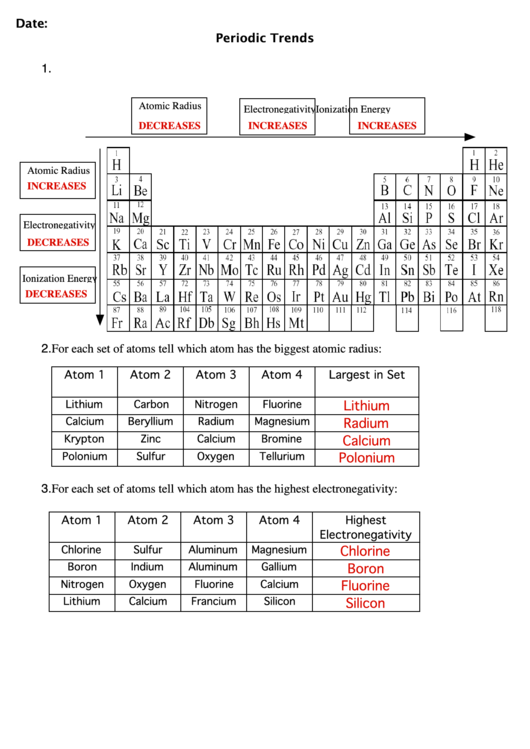
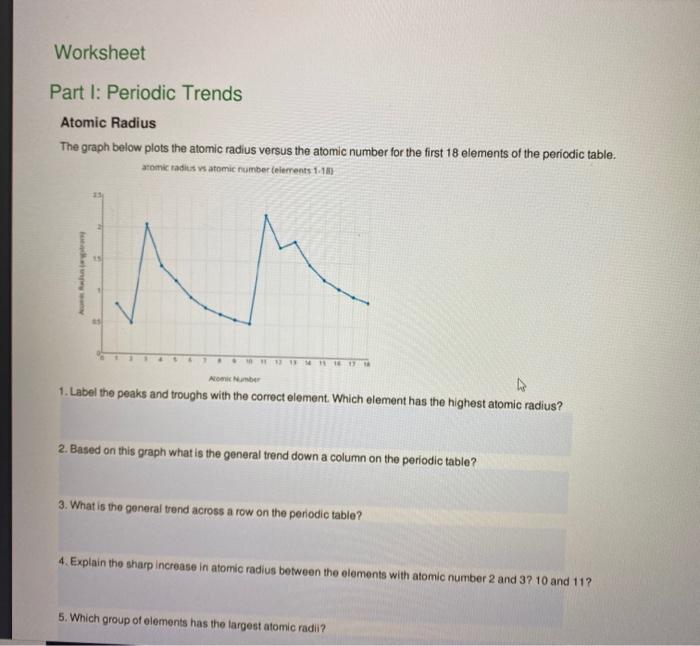
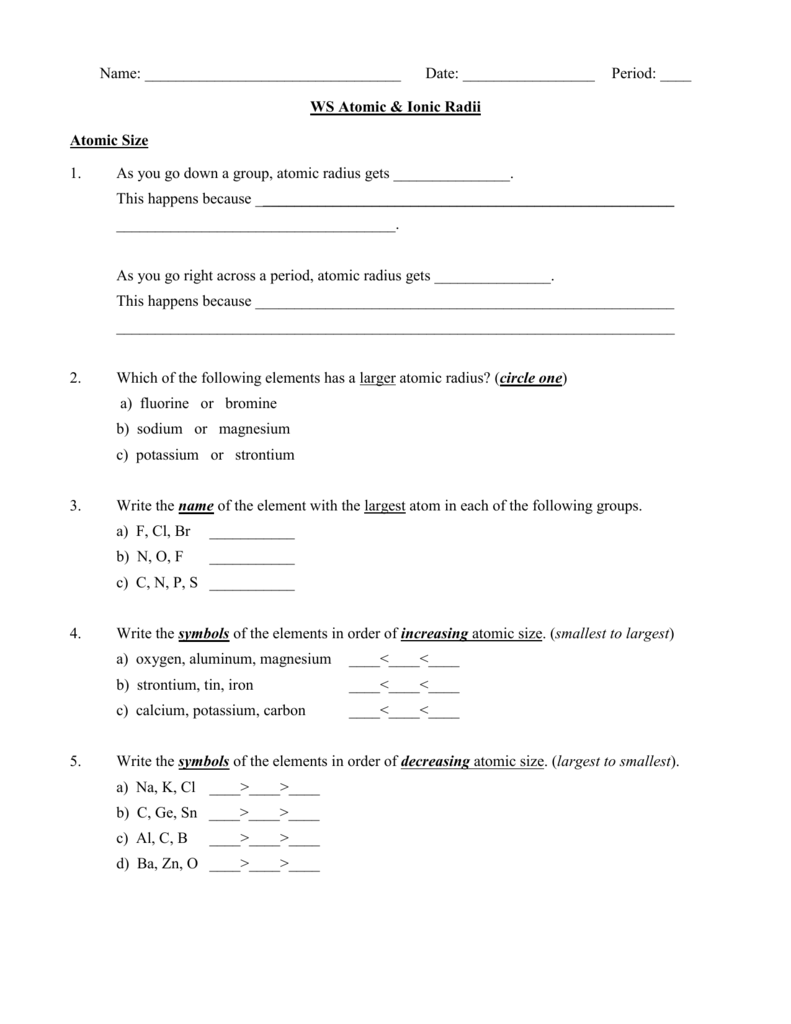
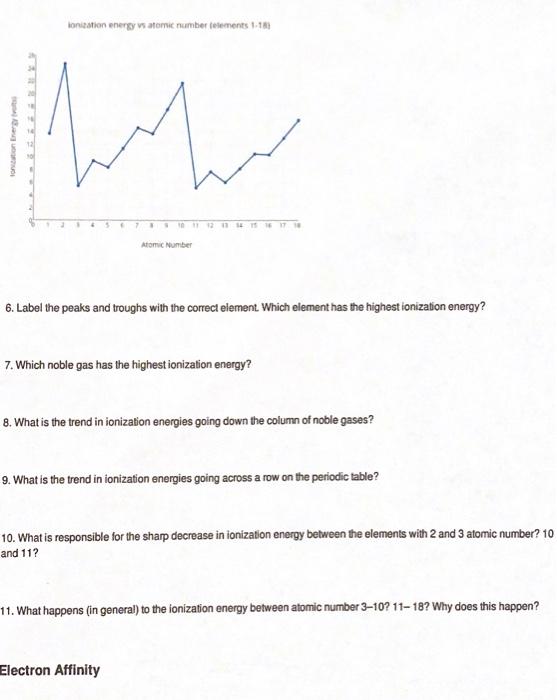
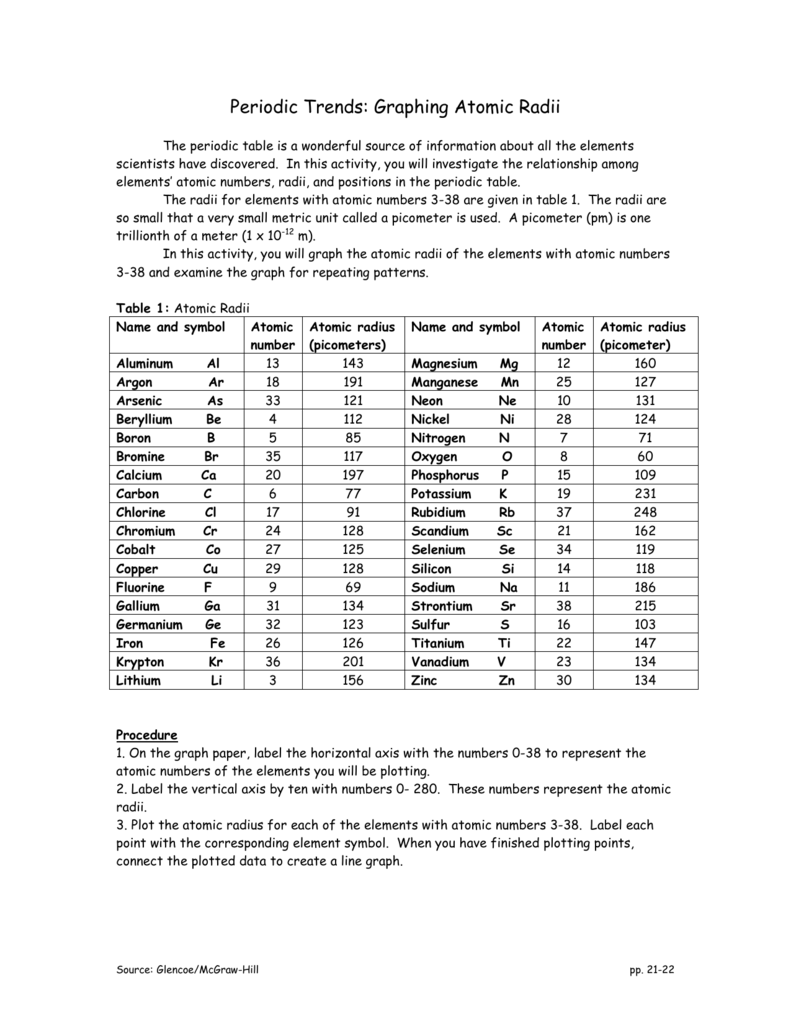
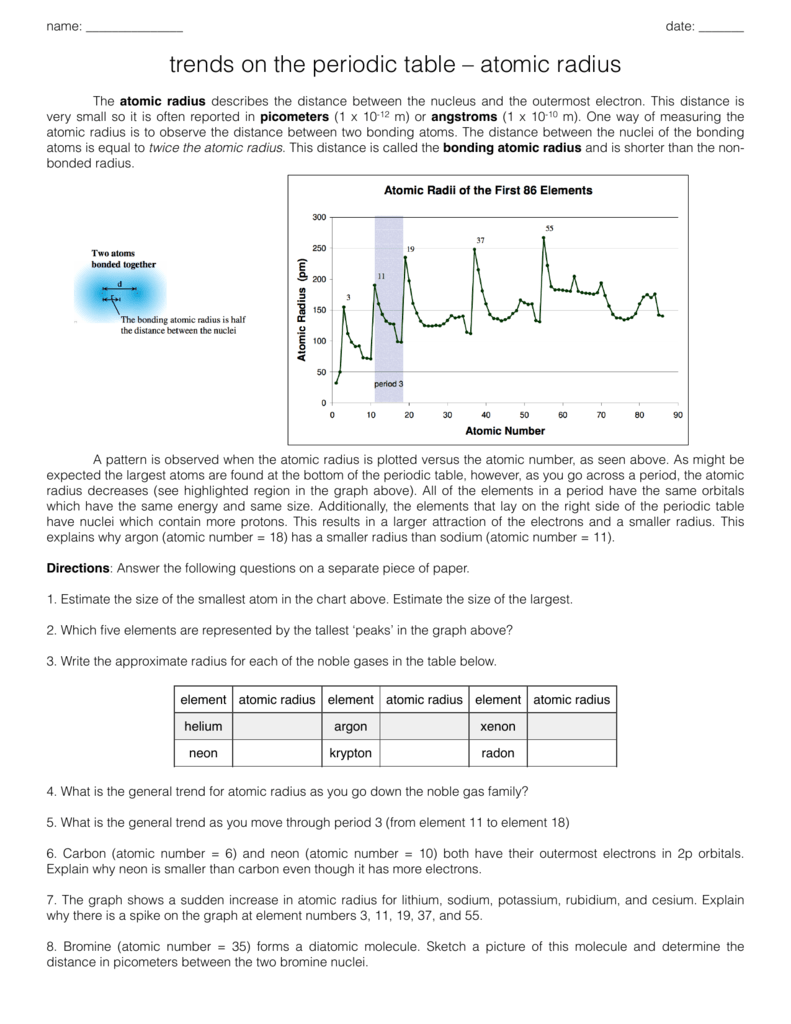
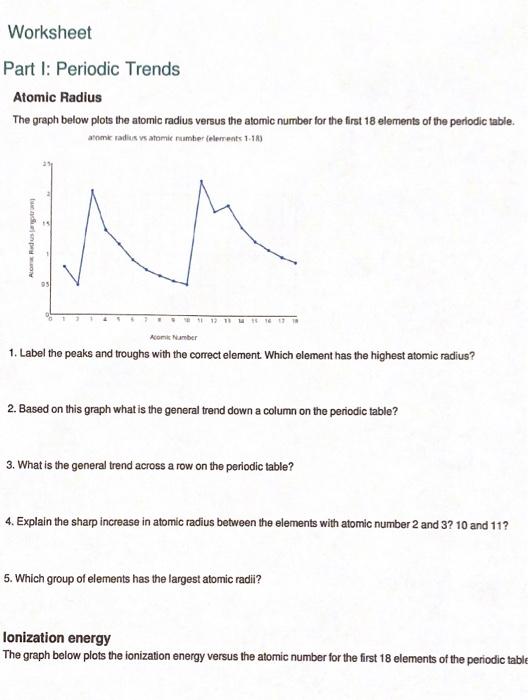
Atomic Radius Worksheet
Atomic Radius Worksheet - What trend in atomic radius do you see as you go down a group/family on the periodic table? On moving to the right of a period, the atomic radius of elements decreases with an increase in the atomic number. Web the following plot shows how atomic radii vary through the periodic table. What makes the sodium atom different from the magnesium atom? Definitions of the atomic radius. One way of measuring the atomic radius is to d observe the distance between bonding atoms. Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. Atomic radii in groups i and ii of the periodic table: The following charts represent the atomic radii trends found on the periodic table. For the second model, students will use the metric ruler to draw vertical “energy bar” lines proportional to ionization energies (figure 4). Using the periodic table, arrange li, ga, ba, cl, and ni in order of increasing atomic radius. A lithium atom, a lithium ion with a charge of 1+ or a sodium atom? Which element has the largest radius? __ use the periodic table to answer the following questions: What makes the sodium atom different from the magnesium atom? What is the general periodic trend for atomic radius? Which element has the smallest radius? What trend in atomic radius do you see as you go down a group/family on the periodic table? What makes the sodium atom different from the magnesium atom? Which of the following is the largest: Worksheets are periodic trends atomic radius name chem work 6 1, work 11, chemistry topic work tests name, university of illinois at urbanachampaign, chapter 8 practice work, work 12, periodic trends work, name work periodic trends period. Some of the worksheets displayed are periodic trends atomic radius name chem work 6 1, work 11, chemistry topic work tests name, university. Al, cl, ga cli ionic radius for each of the following sets of ions, rank them from smallest to largest ionic radius. For the second model, students will use the metric ruler to draw vertical “energy bar” lines proportional to ionization energies (figure 4). Which element has the largest radius? Web the following plot shows how atomic radii vary through. And on moving down the group, the atomic radius increases due to the increase in the number of energy levels. Shielding predominates (rules) which of the following is the smallest: Mg , si b.mg , ca , ba c. How does the atomic radius change within a group and in a period in a periodic table? In this simulation, students. Definitions of the atomic radius. Element m is a metal that forms compounds of the type mx 2,. What trend in atomic radius do you see as you go across a period/row on the periodic table? Web this atomic and ionic radii quiz is meant to assess how well you: Sample of completed atomic radius table. On moving to the right of a period, the atomic radius of elements decreases with an increase in the atomic number. Using the periodic table, arrange li, ga, ba, cl, and ni in order of increasing atomic radius. (a) the covalent atomic radius, r cov, is half the distance between the nuclei of two like atoms joined by a covalent. Circle the atom in each pair that has the largest atomic radius. Some of the worksheets displayed are periodic trends atomic radius name chem work 6 1, work 11, chemistry topic work tests name, university of illinois at urbanachampaign, chapter 8 practice work, work 12, periodic trends work, name work periodic trends period. What is the general group trend for. The questions help students pinpoint the reasoning behind the atomic radius trend and students draw the trend on the periodic table picture provided. The following charts represent the atomic radii trends found on the periodic table. A lithium atom, a lithium ion with a charge of 1+ or a sodium atom? Using the periodic table, arrange li, ga, ba, cl,. Circle the atom in each pair that has the largest atomic radius. (a) the covalent atomic radius, r cov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as cl 2. Write out the complete electron configuration of titanium, and the valence electron configurations of vanadium, chromium, iron,.. Using the periodic table, arrange li, ga, ba, cl, and ni in order of increasing atomic radius. What is the general periodic trend for atomic radius? __ use the periodic table to answer the following questions: And on moving down the group, the atomic radius increases due to the increase in the number of energy levels. Web the following plot shows how atomic radii vary through the periodic table. Size increases down a group. 1 å = 1 × 10 −10 m = 100 pm. The questions help students pinpoint the reasoning behind the atomic radius trend and students draw the trend on the periodic table picture provided. Shielding predominates (rules) which of the following is the smallest: Definitions of the atomic radius. (a) the covalent atomic radius, r cov, is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, such as cl 2. Web arrange ag, pt, mg, c, cu, and si in order of increasing atomic radius. Mg , si b.mg , ca , ba c. What is the general group trend for atomic radius? Sample of completed atomic radius table. What trend in atomic radius do you see as you go across a period/row on the periodic table? Some of the worksheets displayed are periodic trends atomic radius name chem work 6 1, work 11, chemistry topic work tests name, university of illinois at urbanachampaign, chapter 8 practice work, work 12, periodic trends work, name work periodic trends period. A lithium atom, a lithium ion with a charge of 1+ or a sodium atom? Web this atomic and ionic radii quiz is meant to assess how well you: By choosing elements from the periodic table, atoms can be selected. Element element be mg ca sr ba 2.2 2 group 2a atomic number 4 12 20 38 56 atomic radius 1.11 1.60 1.97 2.15 2.17 na mg al si p s cl ar period 3 atomic number 11 12 13 14 15 16 17 18 1 å = 1 × 10 −10 m = 100 pm. What trend in atomic radius do you see as you go down a group/family on the periodic table? Which of the following is the largest: Atomic number for group 2a and for period 3 of the periodic table. For the second model, students will use the metric ruler to draw vertical “energy bar” lines proportional to ionization energies (figure 4). Size increases down a group. What makes the sodium atom different from the magnesium atom? In this simulation, students can investigate the periodic trends of atomic radius, ionization energy, and ionic radius. Web the quiz questions deal with electrostatic forces, atomic models, and radius calculations. Circle the atom in each pair that has the largest atomic radius. Web arrange ag, pt, mg, c, cu, and si in order of increasing atomic radius. Does atomic radius generally increase or decrease across (l to r) a period? Shielding predominates (rules) which of the following is the smallest: The questions help students pinpoint the reasoning behind the atomic radius trend and students draw the trend on the periodic table picture provided. Circle the atom in each pair that has the largest atomic radius.Discovering Trends In The Periodic Table Worksheet Answers
Solved Worksheet Part I Periodic Trends Atomic Radius The
WS Atomic & Ionic Radii
Atomic Radius Worksheet
39 periodic trends atomic radius worksheet answer key Worksheet For Fun
atomic radiuspractice problems
Solved Worksheet Part I Periodic Trends Atomic Radius The
Atomic Radius Worksheet
Periodic Table Trends Worksheet Answer Key Atomic Radius Review Home
periodic trends worksheet draw the trend for atomic radius angelofmine88
Web This Chemistry Homework Page Is Perfect For Students To Analyze The Atomic Radius Trend On The Periodic Table.
The Following Charts Represent The Atomic Radii Trends Found On The Periodic Table.
How Does The Atomic Radius Change Within A Group And In A Period In A Periodic Table?
What Trend In Atomic Radius Do You See As You Go Across A Period/Row On The Periodic Table?
Related Post: