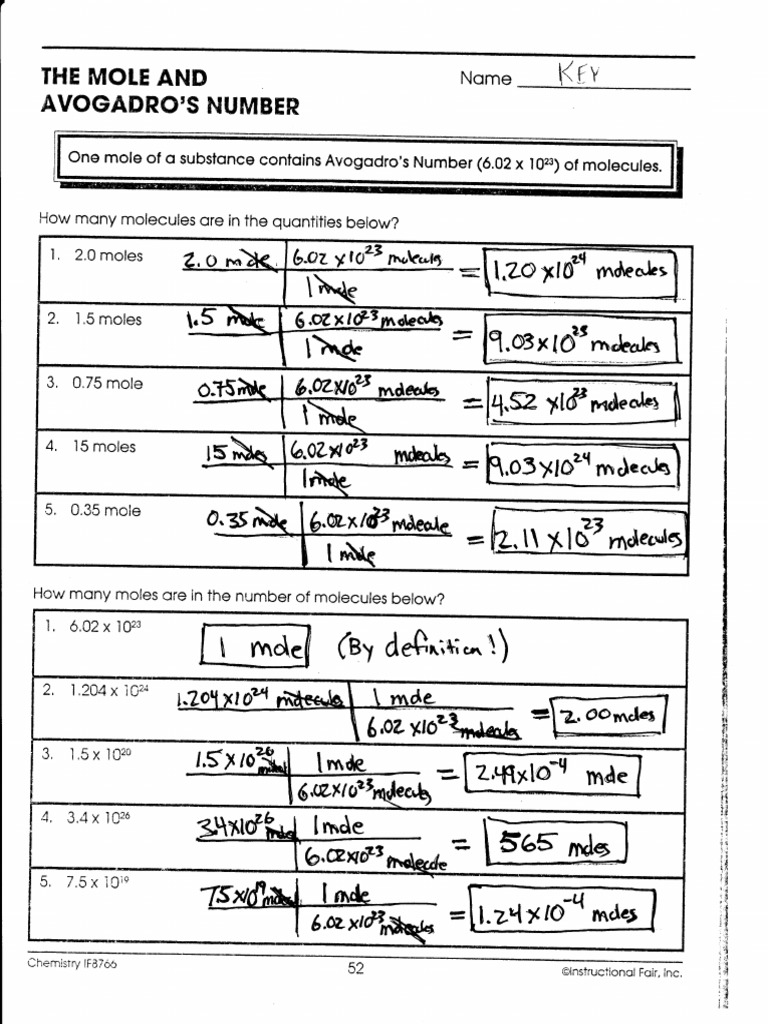
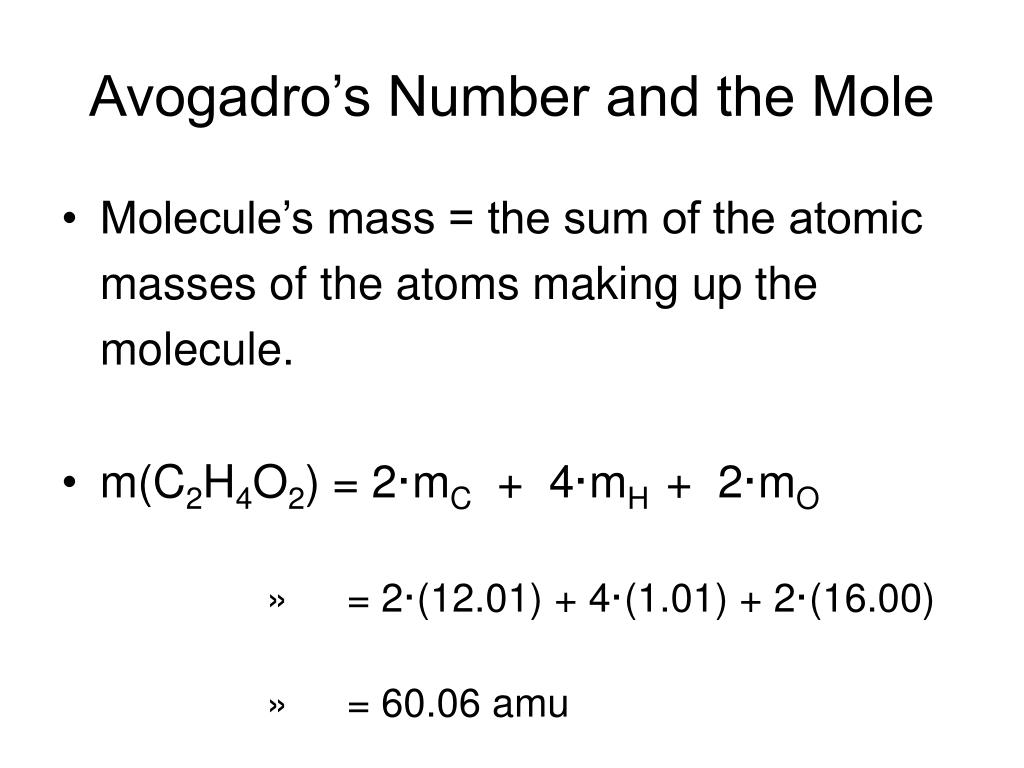Avogadro's Number And The Mole Worksheet
Avogadro's Number And The Mole Worksheet - How many moles of xenon do 5.66 × 1023 atoms equal? What is the molar mass of. A) the mass in mg of mol ag and. Just as a dozen apples is 12. Web using avogadro’s number to convert from molecules or atoms to moles. B) the number of oxygen atoms in 20.5 mol o 2. Some of the worksheets displayed are example exercise atomic mass and avogadros number, avogadros. This senior chemistry lesson package discusses the mole, avogadro’s number, molar. Web mole & avogadro’s number example question. Because this is an awkward number to write over and over again,. Web this senior chemistry lesson package discusses the mole, avogadro’s number, molar mass and provides a lot of practice with the formulas to determine n and the number of. B) the number of oxygen atoms in 20.5 mol o 2. A) the mass in mg of mol ag and. Web showing 8 worksheets for chemistry and avogadros number. What is. Worksheets are the mole and avogadros number, example exercise atomic mass and avogadros numb. Web convert between numbers of atoms, moles, and mass of sample by using avogadro’s number and the appropriate molar mass. Because this is an awkward number to write over and over again,. How many moles of xenon do 5.66 × 1023 atoms equal? Web showing 8. Web mole & avogadro’s number example question. Apples, a mole of apples is 6.022 x 1023 apples. A mole of objects contains avogadro's number, 6.022 x 1023, objects. A) the mass in mg of mol ag and. Web the number 6.02 x 1023 is known as avogadro’s number in honor of an italian professor of physics, amadeo avogadro, who did. Web mole & avogadro’s number example question. How many atoms are there in 1.45 × 10−17 mol of arsenic? K, ag, s) , an ion (e.g. How many ions are there in 0.187 mol of na+ ions? The number 6.022 × 10²³ is known as avogadro's number or. The number 6.022 × 10²³ is known as avogadro's number or. Worksheets are the mole and avogadros number, example exercise atomic mass and avogadros numb. K, ag, s) , an ion (e.g. Solution to this mole & avogadro. For most purposes it is rounded off to 6.022 1023. How many atoms are there in 1.45 × 10−17 mol of arsenic? How many moles of xenon do 5.66 × 1023 atoms equal? Apples, a mole of apples is 6.022 x 1023 apples. Web up to $3 cash back introduction. Web stephen lower simon fraser university learning objectives make sure you thoroughly understand the following essential ideas: Apples, a mole of apples is 6.022 x 1023 apples. Web one mole of a substance is equal to 6.022 × 10 ²³ units of that substance (such as atoms, molecules, or ions). K, ag, s) , an ion (e.g. Web the number 6.02 x 1023 is known as avogadro’s number in honor of an italian professor of physics, amadeo. For most purposes it is rounded off to 6.022 1023. A mole of objects contains avogadro's number, 6.022 x 1023, objects. Web one mole of a substance is equal to 6.022 × 10 ²³ units of that substance (such as atoms, molecules, or ions). Learners use the coefficients in a balanced equation to develop the mole ratios of reactants and. Web the number 6.022 137 1023 is called avogadro’s number. Web stephen lower simon fraser university learning objectives make sure you thoroughly understand the following essential ideas: B) the number of oxygen atoms in 20.5 mol o 2. For most purposes it is rounded off to 6.022 1023. The number 6.022 × 10²³ is known as avogadro's number or. Web this senior chemistry lesson package discusses the mole, avogadro’s number, molar mass and provides a lot of practice with the formulas to determine n and the number of. Worksheets are the mole and avogadros number, example exercise atomic mass and avogadros numb. For most calculations, a rounded value of 6.022 x 1023 (four significant figures) is. The number 6.022. Web stephen lower simon fraser university learning objectives make sure you thoroughly understand the following essential ideas: Some of the worksheets displayed are example exercise atomic mass and avogadros number, avogadros. Web by debbie mcclinton, dr. Web showing 8 worksheets for chemistry and avogadros number. How many atoms are there in 1.45 × 10−17 mol of arsenic? Web this senior chemistry lesson package discusses the mole, avogadro’s number, molar mass and provides a lot of practice with the formulas to determine n and the number of. For most purposes it is rounded off to 6.022 1023. The number 6.022 × 10²³ is known as avogadro's number or. Web mole & avogadro’s number example question. Web one mole of a substance is equal to 6.022 × 10 ²³ units of that substance (such as atoms, molecules, or ions). Web using avogadro’s number to convert from molecules or atoms to moles. This senior chemistry lesson package discusses the mole, avogadro’s number, molar. Web the number 6.02 x 1023 is known as avogadro’s number in honor of an italian professor of physics, amadeo avogadro, who did considerable work on the development of atomic. What is the molar mass of. How many moles of xenon do 5.66 × 1023 atoms equal? B) the number of oxygen atoms in 20.5 mol o 2. A chemical species is either an atom (e.g. Just as a dozen apples is 12. Solution to this mole & avogadro. Web up to $3 cash back introduction. How many ions are there in 0.187 mol of na+ ions? A chemical species is either an atom (e.g. Because this is an awkward number to write over and over again,. Web up to $3 cash back introduction. Web using avogadro’s number to convert from molecules or atoms to moles. What is the molar mass of. Web by debbie mcclinton, dr. Web this senior chemistry lesson package discusses the mole, avogadro’s number, molar mass and provides a lot of practice with the formulas to determine n and the number of. Web stephen lower simon fraser university learning objectives make sure you thoroughly understand the following essential ideas: How many moles of xenon do 5.66 × 1023 atoms equal? Learners use the coefficients in a balanced equation to develop the mole ratios of reactants and products. Apples, a mole of apples is 6.022 x 1023 apples. The number 6.022 × 10²³ is known as avogadro's number or. Web mole & avogadro’s number example question. Web one mole of a substance is equal to 6.022 × 10 ²³ units of that substance (such as atoms, molecules, or ions). This senior chemistry lesson package discusses the mole, avogadro’s number, molar.The Mole And Avogadros Number Worksheet Answers Escolagersonalvesgui
The Mole And Avogadros Number Worksheet Worksheet List
The Mole And Avogadro's Number Worksheet
The Mole And Avogadro's Number Worksheet pivotinspire
PPT Chapter 3 PowerPoint Presentation ID258640
The Mole And Avogadro's Number Worksheet
The Mole And Avogadro's Number Worksheet Answers richinspire
The Mole And Avogadro's Number Worksheet
Moles & Stoichiometry WORKSHEET W1 Avogadro's Number
moles and avogadros number worksheet
Web Showing 8 Worksheets For Chemistry And Avogadros Number.
Web The Number 6.022 137 1023 Is Called Avogadro’s Number.
A Mole Of Objects Contains Avogadro's Number, 6.022 X 1023, Objects.
Web The Number 6.02 X 1023 Is Known As Avogadro’s Number In Honor Of An Italian Professor Of Physics, Amadeo Avogadro, Who Did Considerable Work On The Development Of Atomic.
Related Post: