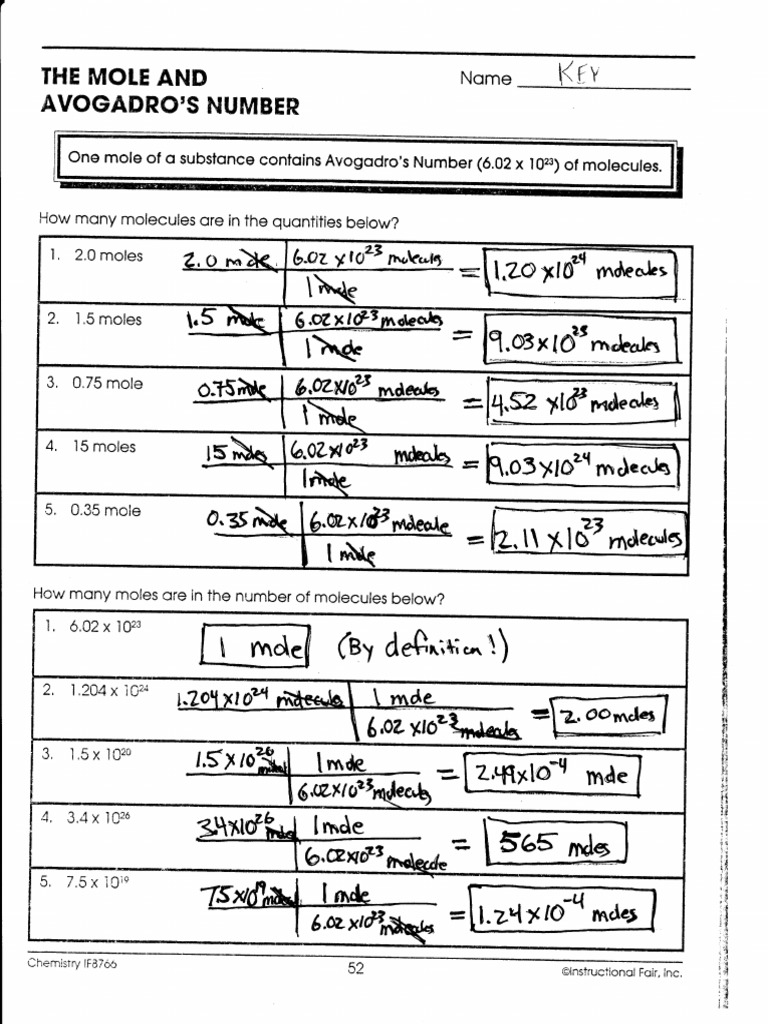
Avogadro's Number Worksheet
Avogadro's Number Worksheet - Web one mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). Web avogadro's number is known to ten significant digits: It is a huge number, far greater in magnitude than we can. Web understanding avogadro's number is a confusing part of chemistry. Web displaying 8 worksheets for avogadro number. How many years would it take the. Each set is available individually or you can buy the entire stoichiometry worksheet bundle at a. Web showing 8 worksheets for chemistry and avogadros number. Web molar mass & avogadro’s number: However, you only need to know it to three significant figures: Determine the atomic mass for the following elements: Web molar mass & avogadro’s number: Some of the worksheets displayed are example exercise atomic mass and avogadros number, avogadros. How many formula units would. Web avogadro's number worksheets have 2 pages of questions converting to and from moles using avogadro's number and includes a full answer key. Name _____ date _____ period _____ 1. How many formula units would. A mole of water molecules is 6.022 x 1023 water molecules. Worksheets are example exercise atomic mass and avogadros number, work mole and avogadros number, chem100. Each set is available individually or you can buy the entire stoichiometry worksheet bundle at a. How many moles of xenon do 5.66 × 1023 atoms equal? How many years would it take the. A mole of water molecules is 6.022 x 1023 water molecules. Na ≈ 6.02 × 1023. Web things to understand about avogadro's number. Web this is part of a larger stoichiometry worksheet bundle that includes 21 sets. 6 rows web up to 24% cash back avogadro's number worksheet. How many years would it take the. Web showing 8 worksheets for chemistry and avogadros number. Worksheets are example exercise atomic mass and avogadros number, work mole and avogadros number, chem100. Web displaying 8 worksheets for avogadros number. Worksheets are example exercise atomic mass and avogadros number, avogadros number practice work, work m. Web avogadro's number is known to ten significant digits: Some of the worksheets displayed are example exercise atomic mass and avogadros number, avogadros. It is a number, just as is dozen, and thus is dimensionless. Web a mole of iron atoms is 6.022 x 1023 iron atoms. Web molar mass & avogadro’s number: Worksheets are example exercise atomic mass and avogadros number, work mole and avogadros number, chem100. Web up to 24% cash back determine the mass of 100 rice grains. How many years would it take you to count a mole of rice grains? How many ions are there in 0.187 mol of na+ ions? Web up to 24% cash back determine the mass of 100 rice grains. Web one mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). Chapter 19 worksheet boltzmann constant: How many years would it take you to count. The nist 2007 value of avogadro's number is 6.022 141 79 ± 0.000. Web up to 24% cash back determine the mass of 100 rice grains. Worksheets are the mole and avogadros number, example exercise atomic mass and avogadros numb. The number 6.022 × 10²³ is known as avogadro's number or. Web using avogadro’s number to convert from molecules or. Determine the number of representative particles in each of the following. Worksheets are the mole and avogadros number, example exercise atomic mass and avogadros numb. Web the number 6.02 x 1023 is known as avogadro’s number in honor of an italian professor of physics, amadeo avogadro, who did considerable work on the development of atomic. Determine the atomic mass for. The number 6.022 × 10²³ is known as avogadro's number or. A chemical species is either an atom (e.g. Na ≈ 6.02 × 1023. Web using avogadro’s number to convert from molecules or atoms to moles. Web displaying 8 worksheets for avogadro number. Web avogadro's number worksheets have 2 pages of questions converting to and from moles using avogadro's number and includes a full answer key. K, ag, s) , an ion (e.g. Web up to 24% cash back determine the mass of 100 rice grains. Some of the worksheets displayed are example exercise atomic mass and avogadros number, avogadros. Worksheets are the mole and avogadros number, example exercise atomic mass and avogadros numb. How many ions are there in 0.187 mol of na+ ions? How many years would it take you to count a mole of rice grains? Web a mole of iron atoms is 6.022 x 1023 iron atoms. The nist 2007 value of avogadro's number is 6.022 141 79 ± 0.000. Web this is part of a larger stoichiometry worksheet bundle that includes 21 sets. It is a number, just as is dozen, and thus is dimensionless. How many formula units would. Web displaying 8 worksheets for avogadros number. However, you only need to know it to three significant figures: How many moles of xenon do 5.66 × 1023 atoms equal? Chapter 19 worksheet boltzmann constant: Web for an element or compound that is composed of molecules, the molar mass in grams is numerically equal to its molecular weight in atomic mass units. It is a huge number, far greater in magnitude than we can. How many atoms are there in 1.45 × 10−17 mol of arsenic? This is part of a larger. Some of the worksheets displayed are example exercise atomic mass and avogadros number, avogadros. Web a mole of iron atoms is 6.022 x 1023 iron atoms. Na = 6.022141527 × 1023. Web up to 24% cash back determine the mass of 100 rice grains. This is part of a larger. Web avogadro's number worksheets have 2 pages of questions converting to and from moles using avogadro's number and includes a full answer key. Web displaying 8 worksheets for avogadro number. Worksheets are example exercise atomic mass and avogadros number, avogadros number practice work, work m. The number 6.022 × 10²³ is known as avogadro's number or. How many atoms are there in 1.45 × 10−17 mol of arsenic? Web using avogadro’s number to convert from molecules or atoms to moles. Web the number 6.02 x 1023 is known as avogadro’s number in honor of an italian professor of physics, amadeo avogadro, who did considerable work on the development of atomic. How many formula units would. Web displaying 8 worksheets for avogadros number. However, you only need to know it to three significant figures: A mole of water molecules is 6.022 x 1023 water molecules.20 Avogadro Number Worksheet Answers Worksheet From Home
The Mole And Avogadro's Number Worksheet pivotinspire
The Mole And Avogadro's Number Worksheet
20 Avogadro Number Worksheet Answers Worksheet From Home
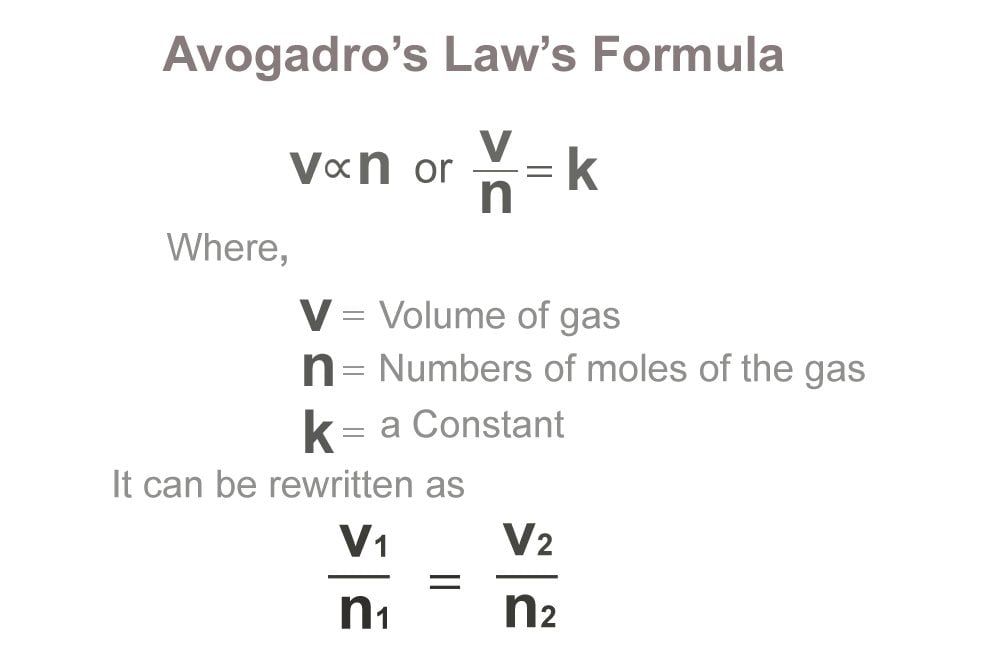
Avogadro's Law Definition, Formula, Equation and Examples
AVOGADRO'S NUMBER WORKSHEET
Mole and Avogadro’s Number Worksheet with Answers Docsity
20 Avogadro Number Worksheet Answers Worksheet From Home
Avogadro's Number Worksheet
30 The Mole And Avogadro's Number Worksheet Answers support worksheet
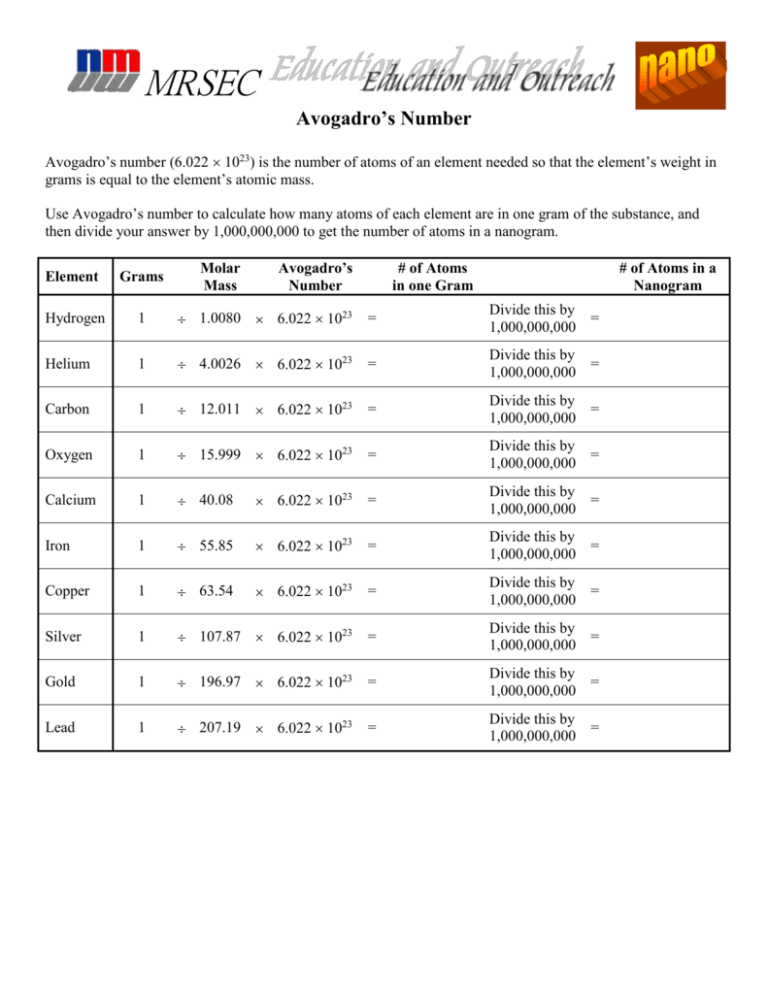
Web For An Element Or Compound That Is Composed Of Molecules, The Molar Mass In Grams Is Numerically Equal To Its Molecular Weight In Atomic Mass Units.
It Is A Huge Number, Far Greater In Magnitude Than We Can.
Each Set Is Available Individually Or You Can Buy The Entire Stoichiometry Worksheet Bundle At A.
Determine The Number Of Representative Particles In Each Of The Following.
Related Post: