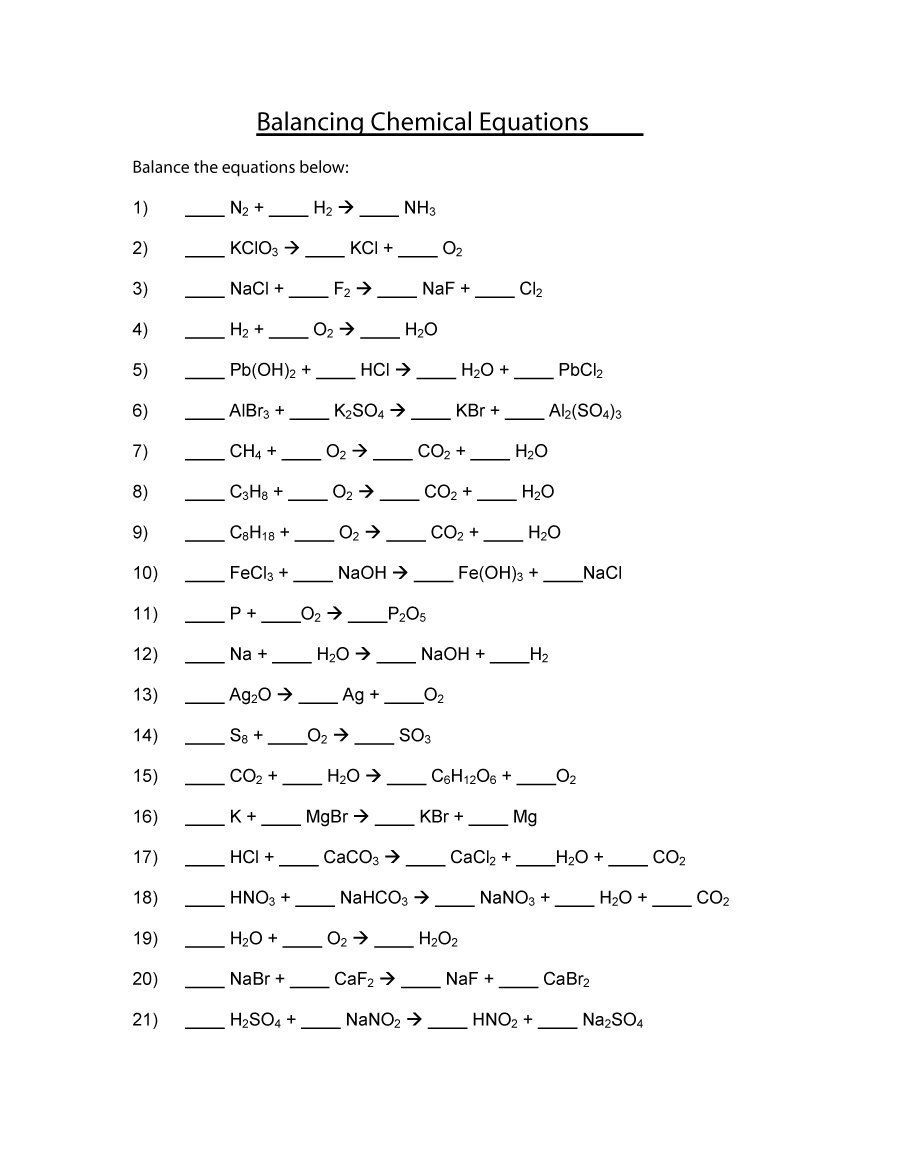
Balancing Chemical Equations Chapter 7 Worksheet 1
Balancing Chemical Equations Chapter 7 Worksheet 1 - Web up to $3 cash back chapter 7 worksheet #1. 1 1 n2 3 h2 2 nh3 2 2 kclo3. 1) 1 n2 + 3 h2 æ 2 nh3 2) 2 kclo3 æ 2 kcl + 3 o2 3) 2 nacl + 1 f2 æ 2 naf + 1 cl2 4) 2 h2 + 1 o2 æ 2 h2o 5) 1 pb(oh)2 + 2 hcl æ 2 h2o +. Web balance the equations below: Web chapter 7 worksheet #1 balancing chemical equations. Balance the following equations and indicate the. Web we have 14 worksheets about chapter 7 worksheet 1 balancing chemical equations including images, pictures, photos, wallpapers, and more. Write the expression for k_ {\mathrm {sp}} k sp for. Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: 1) ____ n2 + ____ h2 æ ____ nh3 2) ____ kclo3 æ ____ kcl + ____ o2. 4 p + ___ o2 = ___ p2o5 3. Writing and balancing chemical reactions 1. Write the balanced chemical equation describing the dissolving of \mathrm {mgco}_3 (s) mgco3(s) in water. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 2 naf. Web up to $3 cash back chapter 7 worksheet #1. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 2 naf + 1 cl 2 4) 2. Web balancing and classifying chemical equations. Web balance the equations below: 1) ____ n2+. 1) ____ n2 + ____ h2 æ ____ nh3. First, balance each of the chemical equations below. Web balance the equations below: 2 na + ____h2o = ____ naoh + h2 4. Writing and balancing chemical reactions 1. 1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 2 naf + 1 cl 2 4) 2 h 2 + 1 o 2 2 h 2o 5). Web balancing and classifying chemical equations. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 2 naf + 1 cl 2 4) 2 h 2 + 1 o 2 2 h 2o 5) 1 pb(oh) 2 + 2 hcl 2 h. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 2 naf + 1 cl 2 4) 2. 1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2. Chemistry add to my workbooks (33) download. 1) ____ n2. Chemistry add to my workbooks (33) download. Web chapter 7 worksheet #1 balancing chemical equations. 1 1 n2 3 h2 2 nh3 2 2 kclo3. 2 ch3(ch2)4ch3 + ____ o2 = 12 co2 + ____h2o 2. Web up to 24% cash back chapter 7 worksheet #1 balancing chemical equations balance the equations below: Chemistry add to my workbooks (33) download. 4 p + ___ o2 = ___ p2o5 3. 1) ____ n2 + ____ h2 æ ____ nh3 2) ____ kclo3 æ ____ kcl + ____ o2. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1. Chemistry add to my workbooks (33) download. Web balance the equations below: Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: 1) ____ n2+ ____ h2æ ____ nh3 2) ____ kclo3æ ____ kcl + ____ o2 3) ____ nacl. Web chapter 7 worksheet #1 balancing chemical equations. 1) _ 1 ___ n 2 + __ 3_ _ h 2 → ___ 2 _ nh 3 2) __ 2 __ kclo 3 → __ 2 __ kcl + __ 3_. Web up to $3 cash back chapter 7 worksheet #1. Writing and balancing chemical reactions 1. Web chapter 7 worksheet #1 balancing chemical equations balance the equations below:. 1) _ 1 ___ n 2 + __ 3_ _ h 2 → ___ 2 _ nh 3 2) __ 2 __ kclo 3 → __ 2 __ kcl + __ 3_. 4 p + ___ o2 = ___ p2o5 3. Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: Writing and balancing chemical reactions 1. Web chapter 7 worksheet #1 balancing chemical equations. 1) ____ n2+ ____ h2æ ____ nh3 2) ____ kclo3æ ____ kcl + ____ o2 3) ____ nacl. First, balance each of the chemical equations below. Web study with quizlet and memorize flashcards containing terms like open system, closed system, all chemical equations have and more. 2) ____ kclo3 æ ____ kcl +. 1) ____ n2 + ____ h2 æ ____ nh3 2) ____ kclo3 æ ____ kcl + ____ o2. 2 na + ____h2o = ____ naoh + h2 4. Chemistry add to my workbooks (33) download. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 2 naf + 1 cl 2 4) 2 h 2 + 1 o 2 2 h 2o 5) 1 pb(oh) 2 + 2 hcl 2 h 2o + 1 pbcl 2. 1 1 n2 3 h2 2 nh3 2 2 kclo3. Write the expression for k_ {\mathrm {sp}} k sp for. Web up to 24% cash back chapter 7 worksheet #1 balancing chemical equations balance the equations below: Web balance the equations below: Write the balanced chemical equation describing the dissolving of \mathrm {mgco}_3 (s) mgco3(s) in water. Then, classify each reaction as synthesis, decomposition,. Web we have 14 worksheets about chapter 7 worksheet 1 balancing chemical equations including images, pictures, photos, wallpapers, and more. Web we have 14 worksheets about chapter 7 worksheet 1 balancing chemical equations including images, pictures, photos, wallpapers, and more. Then, classify each reaction as synthesis, decomposition,. Web up to $3 cash back chapter 7 worksheet #1. Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: First, balance each of the chemical equations below. 2 na + ____h2o = ____ naoh + h2 4. 1) ____ n2 + ____ h2 æ ____ nh3. Web study with quizlet and memorize flashcards containing terms like open system, closed system, all chemical equations have and more. 1) _ 1 ___ n 2 + __ 3_ _ h 2 → ___ 2 _ nh 3 2) __ 2 __ kclo 3 → __ 2 __ kcl + __ 3_. Chemistry add to my workbooks (33) download. 1 1 n2 3 h2 2 nh3 2 2 kclo3. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 2 naf + 1 cl 2 4) 2. Web balance the equations below: Balance the following equations and indicate the. Web chapter 7 worksheet #1 balancing chemical equations balance the equations below: 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 2 naf + 1 cl 2 4) 2 h 2 + 1 o 2 2 h 2o 5) 1 pb(oh) 2 + 2 hcl 2 h 2o + 1 pbcl 2.Chapter 7 Worksheet 1 Balancing Chemical Equations —
49 Balancing Chemical Equations Worksheets [with Answers] Worksheet
Chemistry Balancing Chemical Equations Worksheet Answer Key —
49 Balancing Chemical Equations Worksheets [with Answers]
Balancing Chemical Equations Chapter 7 Worksheet 1
Balancing Chemical Equations Worksheet
49 Balancing Chemical Equations Worksheets [with Answers]
Worksheet More Practice Balancing Equations Balance The Following
Balancing Chemical Equations Chapter 7 Worksheet 1
Balancing Equations Worksheet 1 Answer Key —
Web Up To 24% Cash Back Chapter 7 Worksheet #1 Balancing Chemical Equations Balance The Equations Below:
Web Chapter 7 Worksheet #1 Balancing Chemical Equations.
Web Balancing And Classifying Chemical Equations.
2 Ch3(Ch2)4Ch3 + ____ O2 = 12 Co2 + ____H2O 2.
Related Post:


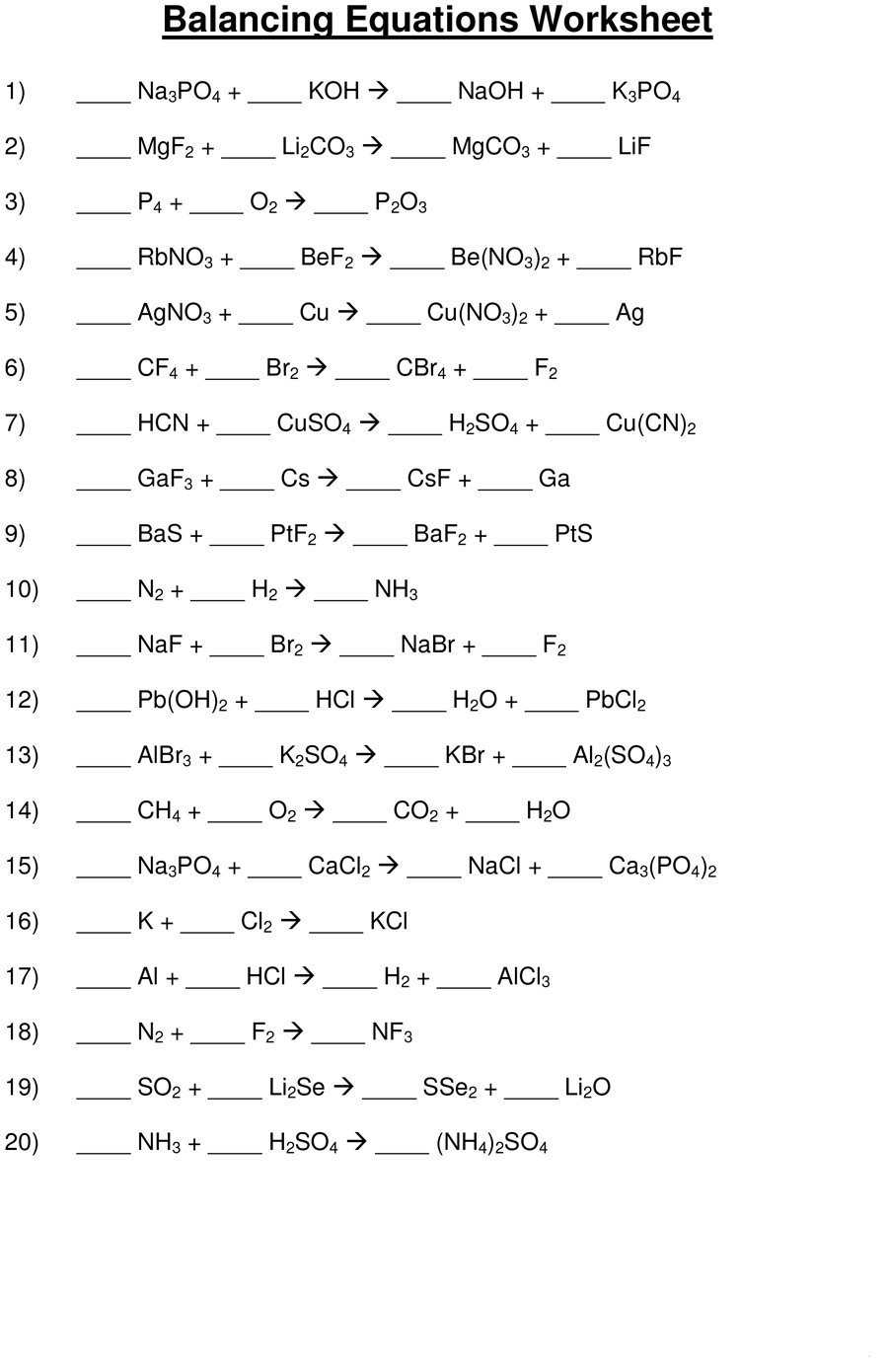
![49 Balancing Chemical Equations Worksheets [with Answers]](http://templatelab.com/wp-content/uploads/2017/01/balancing-equations-07.jpg?w=320)
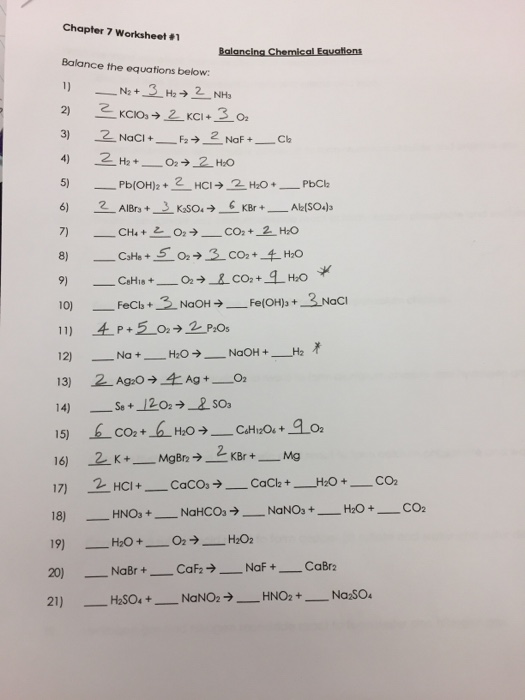

![49 Balancing Chemical Equations Worksheets [with Answers]](https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-08.jpg)