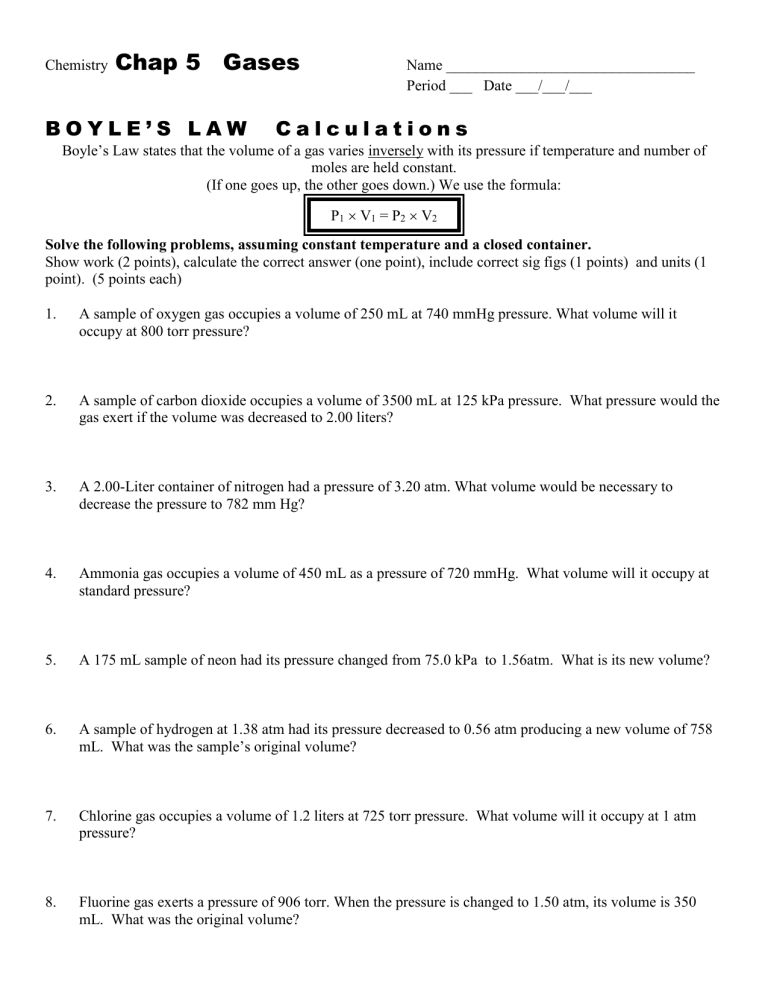
Boyles Law Worksheet
Boyles Law Worksheet - Students will have the opportunity to visually examine the effect of changing the associated variables of pressure, volume, or temperature in each situation. 2 boyles law_essay2_pressure and volume graph experiment 1: Web combine your two proportionality expressions for boyle’s law and for charles’ law into one expression that shows how volume depends on both pressure and. What volume will 50 ml of a gas at 725 torrs occupy if the pressure is increased to 760 torrs? 1 boyles law_essay1_propane pressure and volume experiment 1: These two variables are inversely. (if one goes up, the other. V2= (725 torrs) (50 ml) (760 torrs) answer: Web last updated october 05, 2022. This problem is solved by. 2.00 l of a gas is at 740.0 mmhg pressure. V1= 50 ml p1= 725 torrs p2= 760 torrs. Boyle’s law states that the volume of a gas varies inversely with its pressure if temperature and number of moles is held constant. Web boyle's law worksheet 1. Web round answers to the nearest hundredth. #1 has been done for you. V1= 50 ml p1= 725 torrs p2= 760 torrs. Mmhg to 2.00 atm at constant temperature, what will the volume of the gas sample become? Boyle’s law and charles’s law name______________ boyle’s law: P1v1 = p2v2 v2= p1v1. When ____________ is held constant, the pressure and volume of a gas are ____________. Web students will work through a powerpoint presentation on boyle's law and charles 's law and will record notes and examples on the provided worksheet. P1v1 = p2v2 v2= p1v1. What volume will 50 ml of a gas at 725 torrs occupy if the pressure is. Students will have the opportunity to visually examine the effect of changing the associated variables of pressure, volume, or temperature in each situation. To compress nitrogen at 1 atm from 750 ml to 500 ml, what must the. This problem is solved by. Web up to $3 cash back of 1 boyle’s law practice example 1: If the pressure on. 1 boyles law_essay1_propane pressure and volume experiment 1: Web last updated october 05, 2022. Web boyle's law worksheet 1. _____________________ which variable is plotted on the graph's. Web combine your two proportionality expressions for boyle’s law and for charles’ law into one expression that shows how volume depends on both pressure and. These two variables are inversely. Web last updated october 05, 2022. Mmhg to 2.00 atm at constant temperature, what will the volume of the gas sample become? 1 boyles law_essay1_propane pressure and volume experiment 1: Web zip a sandwich bag nearly closed. This problem is solved by. Web round answers to the nearest hundredth. What is its volume at 760 mmhg pressure? V2= (725 torrs) (50 ml) (760 torrs) answer: Web up to $3 cash back of 1 boyle’s law practice example 1: Record the pairs of data for pressure and propane volume in the table below. 2.00 l of a gas is at 740.0 mmhg pressure. P1v1 = p2v2 v2= p1v1. Insert a straw into the opening and blow through the straw to inflate the bag so that it is a little over half full of air. This problem is solved by. This problem is solved by. #1 has been done for you. What volume will 50 ml of a gas at 725 torrs occupy if the pressure is increased to 760 torrs? When ____________ is held constant, the pressure and volume of a gas are ____________. Web students will work through a powerpoint presentation on boyle's law and charles 's law. Web find boyles law lesson plans and teaching resources. Mmhg to 2.00 atm at constant temperature, what will the volume of the gas sample become? What is its volume at 760 mmhg pressure? 2.00 l of a gas is at 740.0 mmhg pressure. V1= 50 ml p1= 725 torrs p2= 760 torrs. Boyle’s law and charles’s law name______________ boyle’s law: 2 boyles law_essay2_pressure and volume graph experiment 1: Web boyle’s law worksheet name ________________ robert boyle observed the relationship between the pressure and volume for a gas sample. _____________________ which variable is plotted on the graph's. P1v1 = p2v2 v2= p1v1. What is its volume at 760 mmhg pressure? Web combine your two proportionality expressions for boyle’s law and for charles’ law into one expression that shows how volume depends on both pressure and. (if one goes up, the other. This problem is solved by. Web zip a sandwich bag nearly closed. Web round answers to the nearest hundredth. What volume will 50 ml of a gas at 725 torrs occupy if the pressure is increased to 760 torrs? Worksheets are boyles law work with anwer key, boyles law name chem work 14 1, boyles law work, 9 1314 boyles law and. #1 has been done for you. V2= (725 torrs) (50 ml) (760 torrs) answer: Record the pairs of data for pressure and propane volume in the table below. Web students will work through a powerpoint presentation on boyle's law and charles 's law and will record notes and examples on the provided worksheet. Mmhg to 2.00 atm at constant temperature, what will the volume of the gas sample become? Students will have the opportunity to visually examine the effect of changing the associated variables of pressure, volume, or temperature in each situation. V1= 50 ml p1= 725 torrs p2= 760 torrs. Boyle’s law states that the volume of a gas varies inversely with its pressure if temperature and number of moles is held constant. Record the pairs of data for pressure and propane volume in the table below. Web combine your two proportionality expressions for boyle’s law and for charles’ law into one expression that shows how volume depends on both pressure and. Mmhg to 2.00 atm at constant temperature, what will the volume of the gas sample become? If the pressure on a 1.00 l sample of gas is increased from 760. Web students will work through a powerpoint presentation on boyle's law and charles 's law and will record notes and examples on the provided worksheet. _____________________ which variable is plotted on the graph's. 2 boyles law_essay2_pressure and volume graph experiment 1: V2= (725 torrs) (50 ml) (760 torrs) answer: Web find boyles law lesson plans and teaching resources. Web up to $3 cash back of 1 boyle’s law practice example 1: To compress nitrogen at 1 atm from 750 ml to 500 ml, what must the. Boyle’s law and charles’s law name______________ boyle’s law: This problem is solved by. (if one goes up, the other. Web round answers to the nearest hundredth.Chap 12 Boyle's Law Worksheet boyle's Law wksht 12
Boyles Law Worksheets
Boyle's Law Worksheet Name
14 Best Images of Boyle's Law Worksheet Answers Ideal Gas Law
Solved Boyle's Law Name Chem Worksheet 141 Robert Boyle
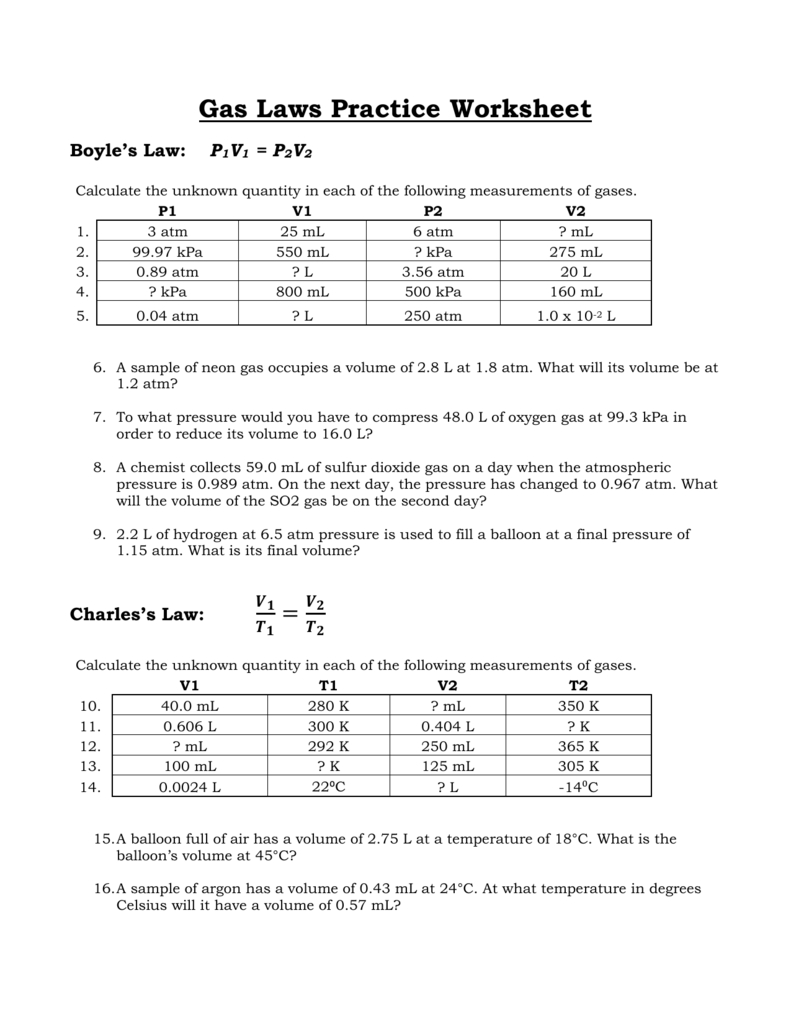
Gas Laws Practice Worksheet Boyles Law —
15 Boyle's Law Worksheet Answers /
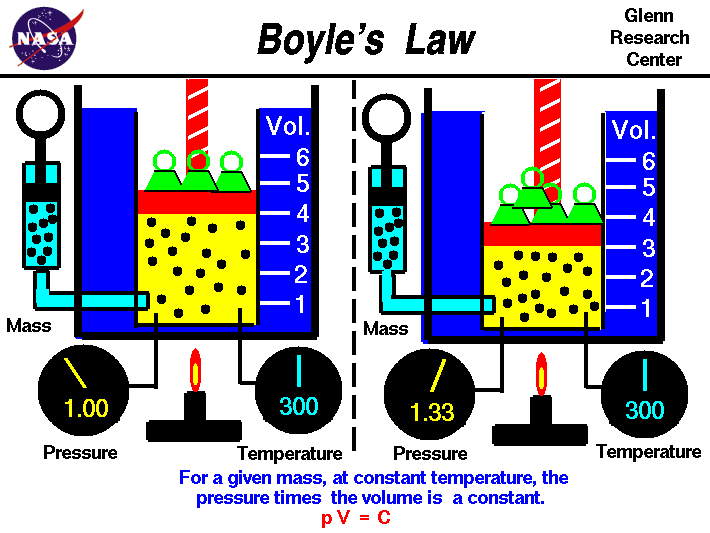
Beginner's Guide to Propulsion Boyle's Law Activity
14 Best Images of Boyle's Law Worksheet Middle School Boyle's Law
Boyles Law Worksheets
What Is Its Volume At 760 Mmhg Pressure?
Web Zip A Sandwich Bag Nearly Closed.
Worksheets Are Boyles Law Work With Anwer Key, Boyles Law Name Chem Work 14 1, Boyles Law Work, 9 1314 Boyles Law And.
Web Boyle's Law Worksheet 1.
Related Post: