Calculating Heat Worksheet
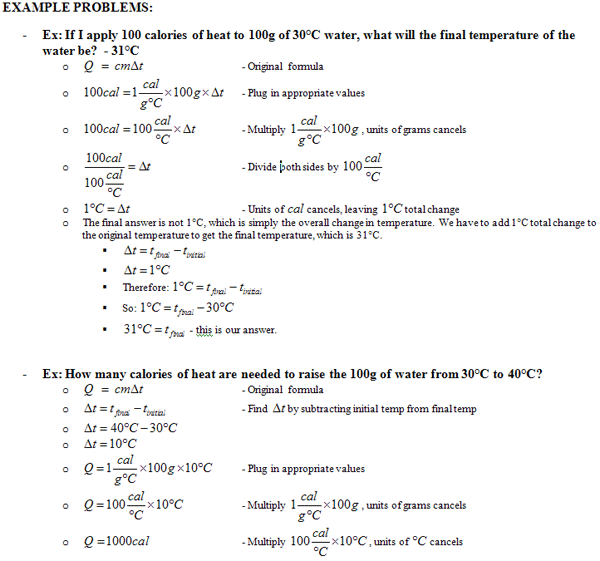
Calculating Heat Worksheet - Show all work including formula. What is the difference in temperature and heat? Thermochemical equations thermochemical equation gives the enthalpy change for the quantities of reactants and products (in moles) in the specified physical. Web work can be defined as a gas changing volume against a constant external pressure. Enthalpy depends upon the amount of substance; If the specific heat of water is 4.18 j/g°c, calculate the amount of heat energy needed to cause this rise in temperature. How many joules of heat are. Web calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6.75×104 joules of heat, and its temperature changes from 32°c to 57°c. Web this worksheet contains problems involving the mcat equation for calculating heat. The general equation for calculating. Worksheets are calculating heat, specific heat calculations work key, name per work introduction to specifi. Web the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. Energy is stored is energy in motion. Web calculating specific heat worksheet name: Heat and heat calculations name 1. Thermochemical equations thermochemical equation gives the enthalpy change for the quantities of reactants and products (in moles) in the specified physical. _____ q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Then, the student is guided through the solution process through the use of a table of relevant variables and a calculation. I.e., it is an extensive property. Web the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. Thermochemical equations thermochemical equation gives the enthalpy change for the quantities of reactants and products (in moles) in the specified physical. If the specific heat of water is 4.18 j/g°c, calculate the. Web q = m × c s × δt. If the specific heat of water is 4.18 j/g°c, calculate the amount of heat energy needed to cause this rise in temperature. The specific heat c is a property of the substance; The first part asks students to recall the formula and to explain the meanings of the variables. C =. Web this quiz and worksheet will help you quickly gauge your knowledge of heat energy and how it is calculated. Web concepts:this worksheet teaches students to perform enthalpy calorimetry calculations. Then, the student is guided through the solution process through the use of a table of relevant variables and a calculation of the temperature change. Web calculate the heat capacity. Web the formula for specific heat looks like this: What is the difference in temperature and heat? Then, the student is guided through the solution process through the use of a table of relevant variables and a calculation of the temperature change. Worksheets are calculating heat, specific heat calculations work key, name per work introduction to specifi. Web calculate the. Web calculating specific heat worksheet name: Web work can be defined as a gas changing volume against a constant external pressure. _____ q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. What is the difference in temperature and heat? Free interactive exercises to practice online or download as pdf to print. The general equation for calculating. Q q is the amount of supplied or subtracted heat (in joules), m m is the mass of. Energy is stored is energy in motion. Web this quiz and worksheet assessment tool provides a quick and effective way you can measure your knowledge of how to calculate heat, temperature and energy. Web this worksheet contains. Enthalpy depends upon the amount of substance; The specific heat c is a property of the substance; Web calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6.75×104 joules of heat, and its temperature changes from 32°c to 57°c. Web heat worksheets and online activities. Web this worksheet contains problems involving the mcat. Web this quiz and worksheet assessment tool provides a quick and effective way you can measure your knowledge of how to calculate heat, temperature and energy. Web this worksheet contains problems involving the mcat equation for calculating heat. Web concepts:this worksheet teaches students to perform enthalpy calorimetry calculations. Heat and heat calculations name 1. Web heat worksheets and online activities. _____ q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Show all work including formula. Web specific heat and heat capacity worksheet directions: Web this quiz and worksheet will help you quickly gauge your knowledge of heat energy and how it is calculated. Web heat worksheets and online activities. Web showing 8 worksheets for calculating heat. Use q = (m)(cp))(δt) to solve the following problems. Energy is stored is energy in motion. Web q = m × c s × δt. Web this worksheet contains problems involving the mcat equation for calculating heat. What is the difference in temperature and heat? Enthalpy depends upon the amount of substance; Q = mc∆t and requires conversion of gram to moles and. The first part asks students to recall the formula and to explain the meanings of the variables. Thermochemical equations thermochemical equation gives the enthalpy change for the quantities of reactants and products (in moles) in the specified physical. Web the same amount of heat is given up when 1.0g of water vapor is changed to liquid water. Web the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. Its si unit is j/(kg ⋅ ⋅. The general equation for calculating. Heat and heat calculations name 1. Web work can be defined as a gas changing volume against a constant external pressure. Web this quiz and worksheet assessment tool provides a quick and effective way you can measure your knowledge of how to calculate heat, temperature and energy. Web the formula for specific heat looks like this: Web the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. I.e., it is an extensive property. Worksheets are calculating heat, specific heat calculations work key, name per work introduction to specifi. Then, the student is guided through the solution process through the use of a table of relevant variables and a calculation of the temperature change. 100.0 ml of 4.0°c water is heated until its temperature is 37°c. Heat is the transfer of energy due to temperature differences. Web heat worksheets and online activities. If the specific heat of water is 4.18 j/g°c, calculate the amount of heat energy needed to cause this rise in temperature. Enthalpy depends upon the amount of substance; Web q = m × c s × δt. The specific heat c is a property of the substance; Web concepts:this worksheet teaches students to perform enthalpy calorimetry calculations. What is the difference in temperature and heat?Calculating Specific Heat Worksheet
Calculating Specific Heat Worksheet Worksheet List
12 Best Images of Heat Worksheet 1 Specific Heat Calculations
Heat online worksheet for Grade 1
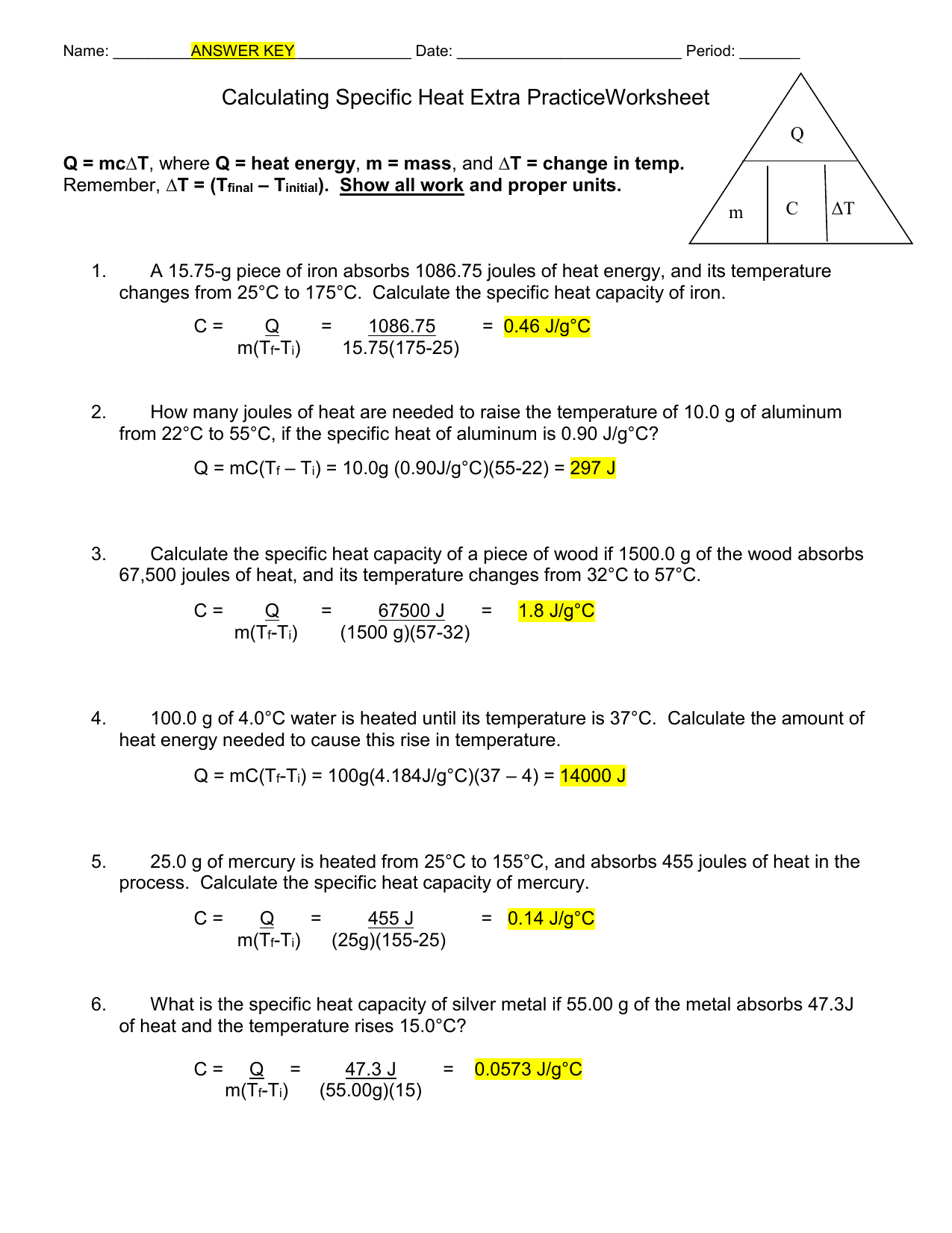
Calculating Specific Heat Extra Practice Worksheet
30 Calculating Specific Heat Worksheet Education Template
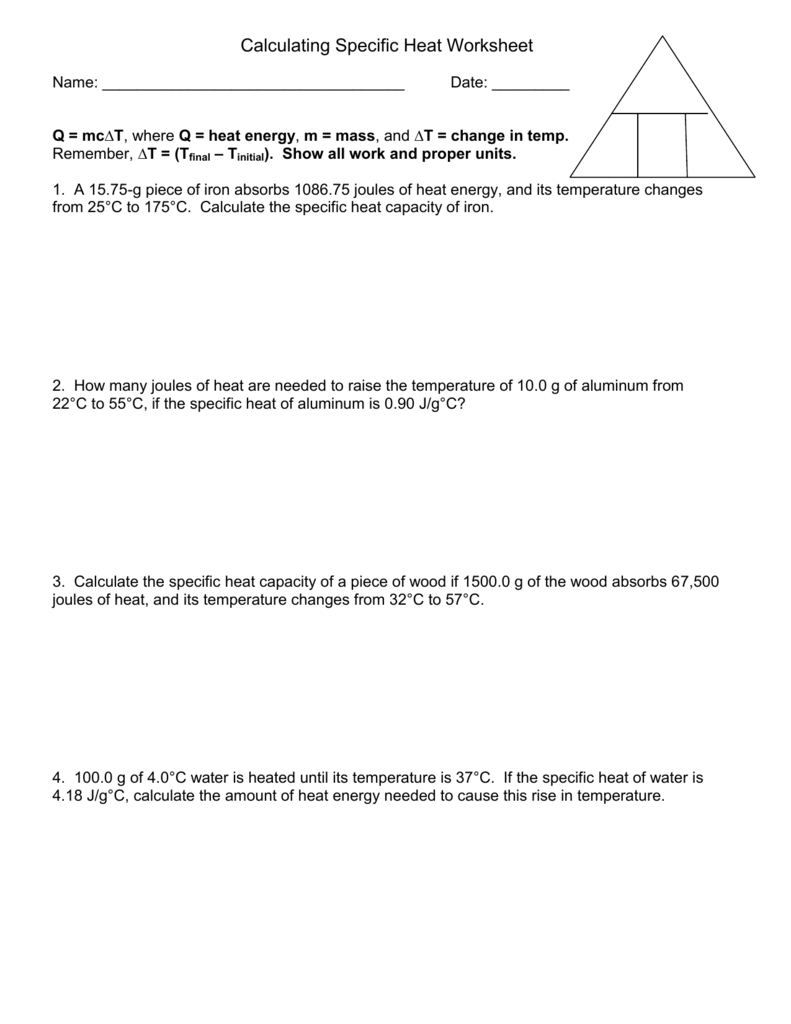
Calculating Specific Heat Worksheet
Calculating Specific Heat Worksheet Worksheets For Kindergarten
30 Calculating Specific Heat Worksheet Education Template
30 Calculating Specific Heat Worksheet Education Template
Its Si Unit Is J/(Kg ⋅ ⋅.
The First Part Asks Students To Recall The Formula And To Explain The Meanings Of The Variables.
Q = Mc∆T And Requires Conversion Of Gram To Moles And.
Web The Same Amount Of Heat Is Given Up When 1.0G Of Water Vapor Is Changed To Liquid Water.
Related Post: