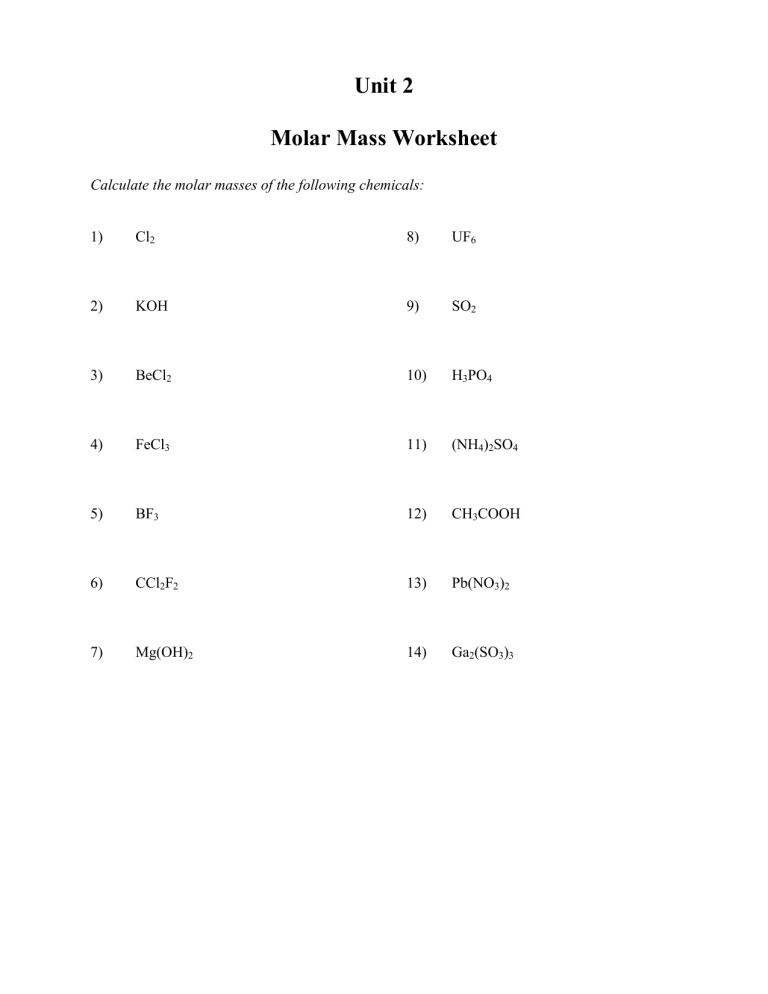
Calculating Molarity Worksheet Answer Key
Calculating Molarity Worksheet Answer Key - Web solutions molarity worksheet name: Show all work and circle your final answer. 0.444 mol of cocl 2 in 0.654 l of solution. Perfect for classwork, homework, extra practice, or as examples for students in a distance learning. Web practice calculating concentration in molarity with this 12 problem worksheet. A complete answer key is provided. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? Web molarity and molality practice worksheet. Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. A complete answer key is provided. Web molarity and molality practice worksheet. Web there are a number of ways to express the relative amounts of solute and solvent in a solution. Web molarity problems worksheet use m or mol/l as unit for molarity. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l. Web determine the molarity for each of the following solutions: Web the molarity of a solution is calculated by dividing the moles of solute by the volume of the solution in liters. 98.0 g of phosphoric acid, h 3 po 4, in 1.00 l of solution. Perfect for classwork, homework, extra practice, or as examples for students in a distance. When we dissolve a cube of sugar in one cup of water, we create a homogeneous mixture. A complete answer key is provided at the end. Molarity = moles of solute liters of solution 3.00 moles. Web determine the molarity for each of the following solutions: Web what determines the concentration of a solution? Show all work and circle your final answer. This worksheet provides many examples for students to practice calculations involving molarity & molality. Web molarity problems worksheet use m or mol/l as unit for molarity. 0.444 mol of cocl 2 in 0.654 l of solution. Perfect for classwork, homework, extra practice, or as examples for students in a distance learning. Web molarity = _____ problems: Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. The volume and molarity of the solution are specified, so the amount (mol) of solute is easily computed as demonstrated in example 6.1.3: This worksheet provides students practice finding calculations finding different. 98.0 g of phosphoric acid,. This worksheet provides students practice finding calculations finding different. Web determine the molarity for each of the following solutions: Web this worksheet provides many examples for students to practice calculations involving molarity & molality. Show all work and circle your final answer. Remember that 1 liter = 1000 ml. Such mixture is called a. This worksheet provides students practice finding calculations finding different. Web molarity and molality practice worksheet. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. A complete answer key is provided. A complete answer key is provided. Web this worksheet provides many examples for students to practice calculations involving molarity & molality. It's important to note that the volume is measured in liters of solution. Do not confuse m, l, and ml! To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution. Web what determines the concentration of a solution? Perfect for classwork, homework, extra practice, or as examples for students in a distance learning. Web solutions molarity worksheet name: This worksheet provides many examples for students to practice calculations involving molarity. Web practice calculating concentration in molarity with this 12 problem worksheet. Web there are a number of ways to express the relative amounts of solute and solvent in a solution. Web practice calculating concentration in molarity with this 12 problem worksheet. It's important to note that the volume is measured in liters of solution. Web this worksheet provides many examples for students to practice calculations involving molarity & molality. Show all. What is the molarity of a solution made by dissolving 3.00 moles of nacl in enough water to make 6.00 liters of solution? Web the molarity of a solution is calculated by dividing the moles of solute by the volume of the solution in liters. A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution is prepared with a total volume of 0.0750\,\text {l} 0.0750l. Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Web what determines the concentration of a solution? 0.444 mol of cocl 2 in 0.654 l of solution. Remember that 1 liter = 1000 ml. It's important to note that the volume is measured in liters of solution. Web determine the molarity for each of the following solutions: When we dissolve a cube of sugar in one cup of water, we create a homogeneous mixture. Web molarity problems worksheet use m or mol/l as unit for molarity. This worksheet provides many examples for students to practice calculations involving molarity & molality. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. A complete answer key is provided. Show all work and circle your final answer. Web molarity = _____ problems: Perfect for classwork, homework, extra practice, or as examples for students in a distance learning. Molarity = moles of solute liters of solution 3.00 moles. Moles of solute 4.00 m = 12.0 l moles of solute = 48.0 mol 2. Web there are a number of ways to express the relative amounts of solute and solvent in a solution. What is the molarity of a solution containing 325 g of nacl. This worksheet provides many examples for students to practice calculations involving molarity. This worksheet provides students practice finding calculations finding different. Web solutions molarity worksheet name: A complete answer key is provided. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. Remember that 1 liter = 1000 ml. Web what determines the concentration of a solution? Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Web determine the molarity for each of the following solutions: Perfect for classwork, homework, extra practice, or as examples for students in a distance learning. Show all work and circle your final answer. A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution is prepared with a total volume of 0.0750\,\text {l} 0.0750l. Web molarity and molality practice worksheet. Web molarity problems worksheet use m or mol/l as unit for molarity.Molarity Problems Worksheet Answer Key Martin Lindelof
Molarity, Molality, Normality, and Mass Percent Worksheet II Answer Key
Molarity Worksheet 1 Answer Key Chemistry
31 Molarity Worksheet Answer Key Education Template
Molarity Worksheet Answer Key
️Molarity Practice Worksheet Free Download Goodimg.co
Molarity Worksheet Answer Key
Molarity Worksheet 1 worksheet
chemistry molarity worksheet answers
Molarity Worksheet Answer Key
Calculate The Molarity Of A Solution Which Contains 0.40 Mol Of A Substance Dissolved In 1.6 L Of A Solution.
It's Important To Note That The Volume Is Measured In Liters Of Solution.
0.444 Mol Of Cocl 2 In 0.654 L Of Solution.
What Is The Molarity Of A Solution Made By Dissolving 3.00 Moles Of Nacl In Enough Water To Make 6.00 Liters Of Solution?
Related Post: