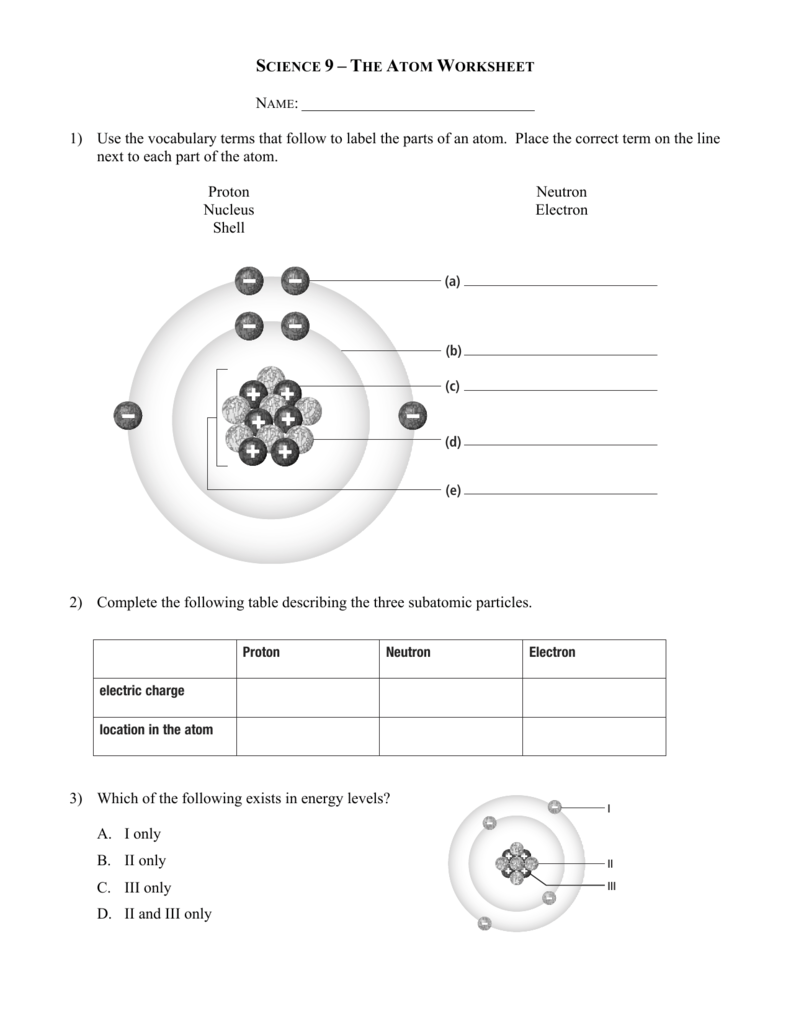
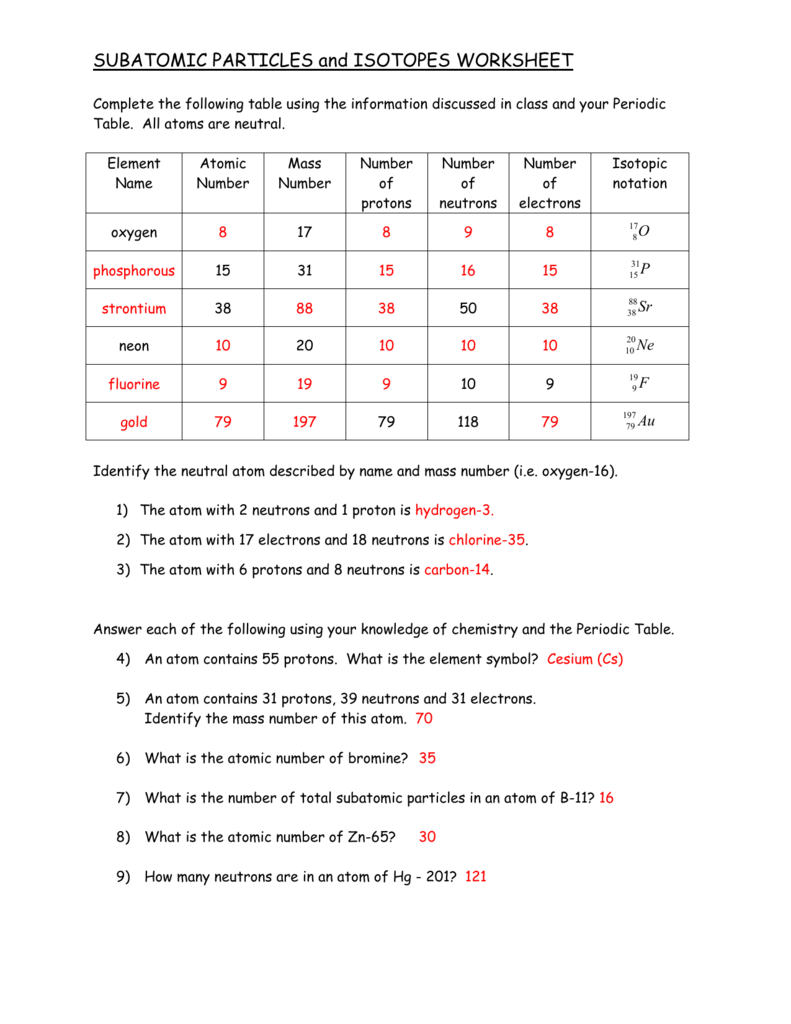
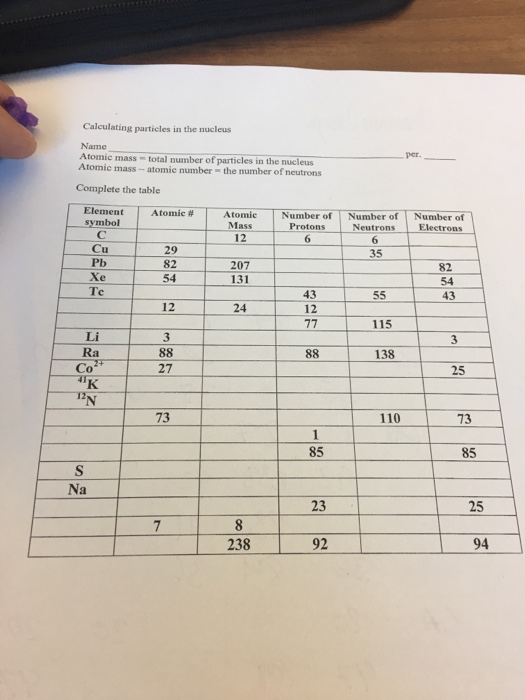
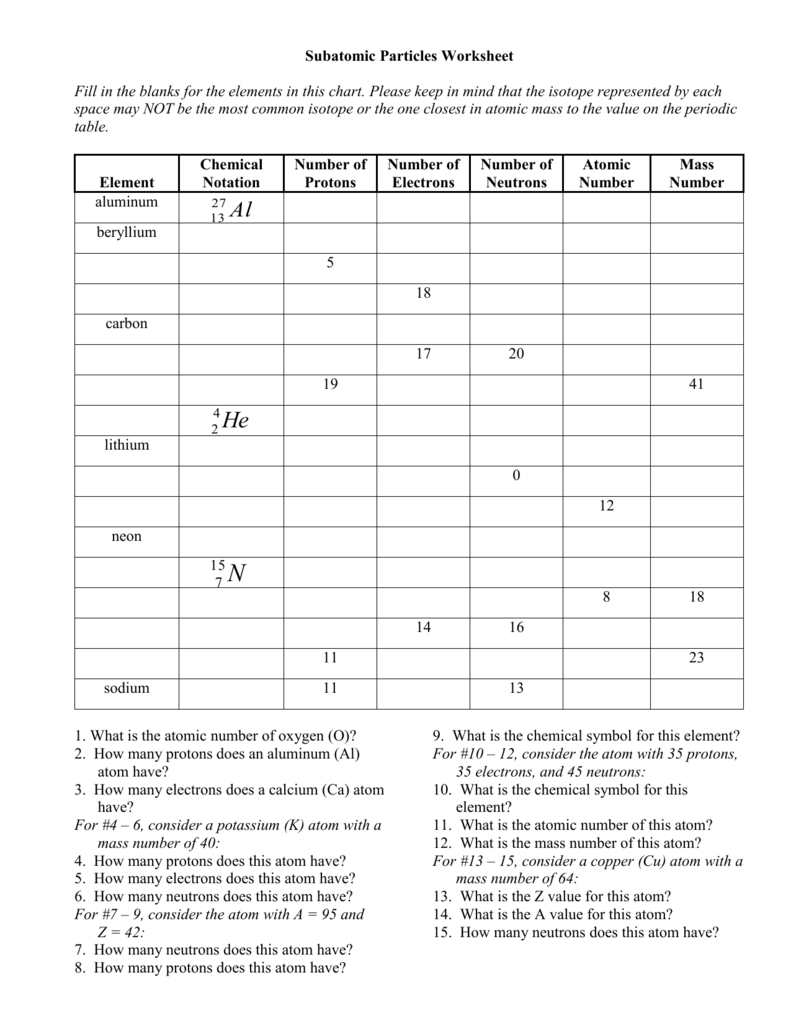
Calculating Particles In The Nucleus Worksheet
Calculating Particles In The Nucleus Worksheet - Web chrome_reader_mode enter reader function. In the case of subatomic particles, the subscript still indicates the charge but will not correspond to the number of protons. Web showing 8 worksheets for calculating particals in the nucleus. Describe the general arrangement of subatomic particles in the atom electrons surround the nucleus; Web to calculate the numbers of subatomic particles in an atom use its atomic number and mass number: Number of protons = atomic number number of electrons = atomic number. Web chrome_reader_mode enter reader mode. Give the symbol of each isotope with the mass number as the superscript and. The boxes on the right represent the daughter product —the atom produced by radioactive decay—and the emitted alpha particle. Calculate the atomic mass of an element given its isotopes Web chrome_reader_mode enter reader function. What you see is an equation that shows the original uranium atom on the left. Web calculate the mass number of each isotope by adding together the numbers of protons and neutrons. Describe the general arrangement of subatomic particles in the atom electrons surround the nucleus; This definition can be represented in an equation, as. Web the mass number of an atom is equal to the total number of protons and neutrons contained in its nucleus. What you see is an equation that shows the original uranium atom on the left. What contribution did these scientists make to atomic models of the atom? Nucleons (particles within the nucleus): Atomic nucleus with an atomic number up. Web describe the composition and size of an atomic nucleus; Many entities can be involved in nuclear reactions. Web together the more neutrons are needed to bind the nucleus together. This definition can be represented in an equation, as shown below. Web up to 24% cash back these particles are summary of the parts of an atom: Worksheets are km 654e 20150109102424, , , nuclear physics work answers, protons neutro. Web together the more neutrons are needed to bind the nucleus together. Nuclei with higher atomic numbers have more neutrons to protons. Web chrome_reader_mode enter reader mode. Web types of particles in nuclear reactions. Web the subscript (79) indicates the charge on the nucleus (the number of protons for a nuclide). Describe the general arrangement of subatomic particles in the atom electrons surround the nucleus; The boxes on the right represent the daughter product —the atom produced by radioactive decay—and the emitted alpha particle. In order to calculate the number of neutrons you must. Web the mass number is used to calculate the number of o in one atom of an element. In the case of subatomic particles, the subscript still indicates the charge but will not correspond to the number of protons. Give the symbol of each isotope with the mass number as the superscript and. Web describe the composition and size of. The most common are protons, neutrons, alpha particles, beta particles,. And charge location relative mass the nucleus is name the nucleus at the symbol of every atom is a. This definition can be represented in an equation, as shown below. Some of the worksheets for this concept are km 654e 20150109102424, , , nuclear. Describe the general arrangement of subatomic. Web the subscript (79) indicates the charge on the nucleus (the number of protons for a nuclide). Web the mass number is used to calculate the number of o in one atom of an element. In order to calculate the number of neutrons you must subtract the from the mqss give the. Use a nuclear symbol to express the composition. Web chrome_reader_mode enter reader mode. In the top left box, write the mass number of the daughter product and press “enter” on your. Explain why the number of neutrons is greater than protons in heavy nuclei; Many entities can be involved in nuclear reactions. Nucleons (particles within the nucleus): Web the mass number is used to calculate the number of o in one atom of an element. Explain why the number of neutrons is greater than protons in heavy nuclei; In the top left box, write the mass number of the daughter product and press “enter” on your. Calculate the atomic mass of an element given its isotopes Web. Nuclei with higher atomic numbers have more neutrons to protons. Web the subscript (79) indicates the charge on the nucleus (the number of protons for a nuclide). Web chrome_reader_mode enter reader function. Web the mass number is used to calculate the number of o in one atom of an element. Explain why the number of neutrons is greater than protons in heavy nuclei; Web the mass number of an atom is equal to the total number of protons and neutrons contained in its nucleus. The number of neutrons needed to create a stable nucleus increase more than the number of protons. Web calculate the mass number of each isotope by adding together the numbers of protons and neutrons. Web give the isotope symbol and number of neutrons in one atom of the following elements. Calculate the atomic mass of an element given its isotopes Nucleons (particles within the nucleus): Some of the worksheets for this concept are km 654e 20150109102424, , , nuclear. Give the symbol of each isotope with the mass number as the superscript and. Use a nuclear symbol to express the composition of an atomic nucleus; Describe the general arrangement of subatomic particles in the atom electrons surround the nucleus; Web up to 24% cash back these particles are summary of the parts of an atom: Web write a balanced equation for each of the following nuclear reactions: Number of protons = atomic number number of electrons = atomic number. Many entities can be involved in nuclear reactions. Web describe the composition and size of an atomic nucleus; Some of the worksheets for this concept are km 654e 20150109102424, , , nuclear physics work answers, protons neutrons and electrons practice work, chemistry work atomic number and mass number, atomic structure work. The number of neutrons needed to create a stable nucleus increase more than the number of protons. Use a nuclear symbol to express the composition of an atomic nucleus; Some of the worksheets for this concept are km 654e 20150109102424, , , nuclear. Web the subscript (79) indicates the charge on the nucleus (the number of protons for a nuclide). Web chrome_reader_mode enter reader mode. Atomic nucleus with an atomic number up to twenty has almost equal number of protons and neutrons. In order to calculate the number of neutrons you must subtract the from the mqss give the. What contribution did these scientists make to atomic models of the atom? Web calculate the mass number of each isotope by adding together the numbers of protons and neutrons. Nuclei with higher atomic numbers have more neutrons to protons. What you see is an equation that shows the original uranium atom on the left. Web types of particles in nuclear reactions. Worksheets are km 654e 20150109102424, , , nuclear physics work answers, protons neutro. Web showing 8 worksheets for calculating particals in the nucleus. Web up to 24% cash back these particles are summary of the parts of an atom:Parts Of An Atom Worksheet
Subatomic Particle Worksheet Answers Educational Worksheet
calculating particles in the nucleus worksheet
Calculating Particles In The Nucleus Worksheet
Calculating Particles In The Nucleus Worksheet
Atomic Structure Worksheet Answers Key Basic Atomic Structure
48+ calculating particles in the nucleus answer key CahraAllysa
Chemistry Unit 1 Worksheet 6
The Cell Nucleus Grade 1 to 12 Reproducible Student Worksheets
Subatomic Particles Worksheet Answers
Many Entities Can Be Involved In Nuclear Reactions.
Give The Symbol Of Each Isotope With The Mass Number As The Superscript And.
Web Chrome_Reader_Mode Enter Reader Function.
Calculate The Atomic Mass Of An Element Given Its Isotopes
Related Post: