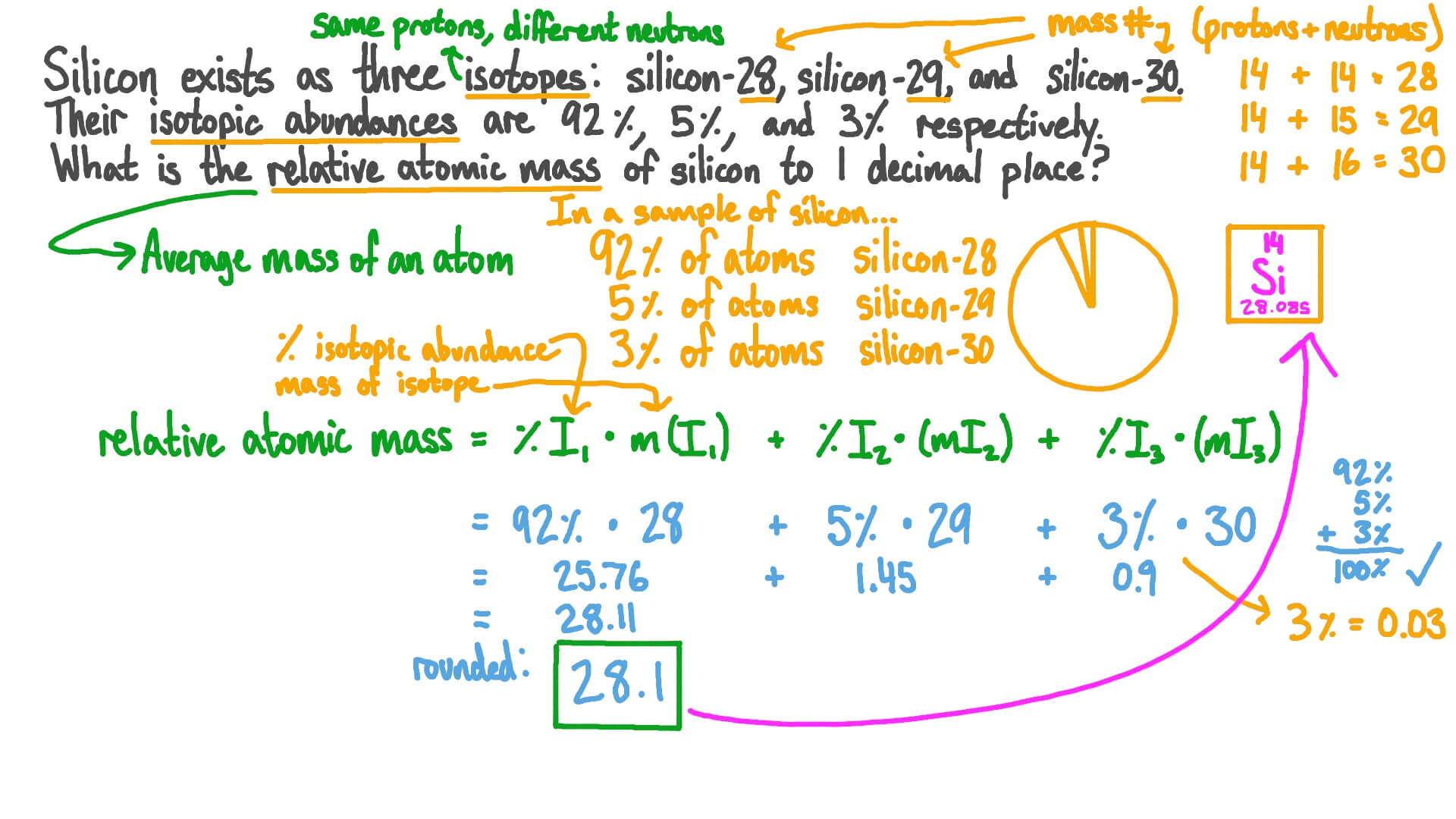
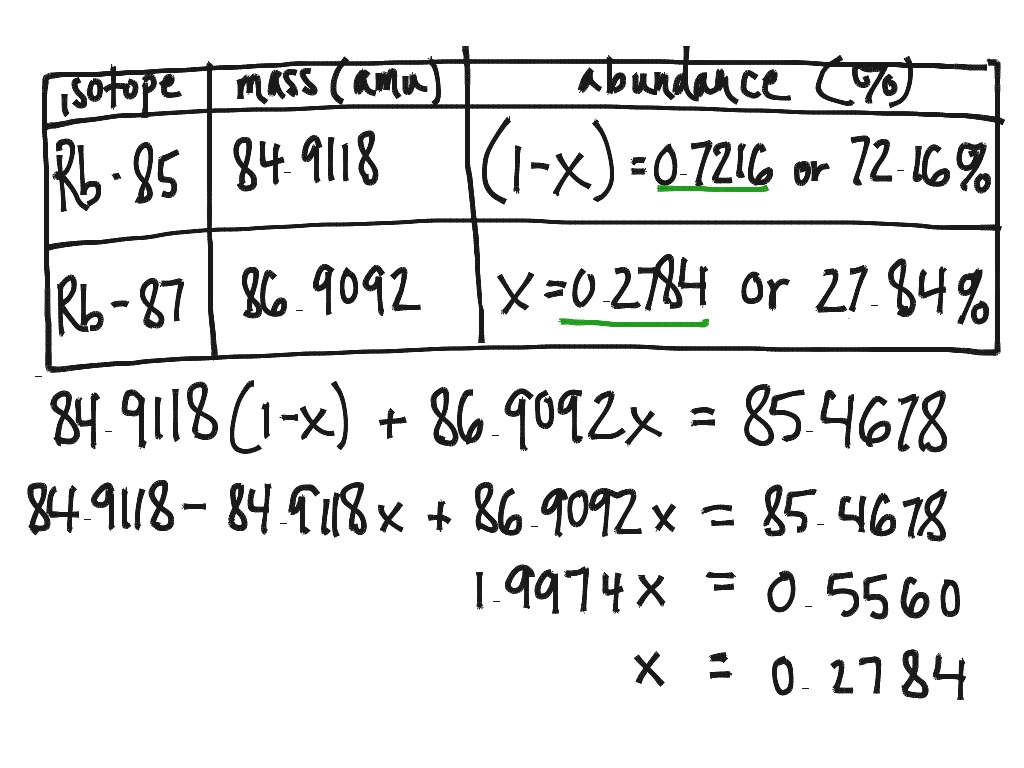
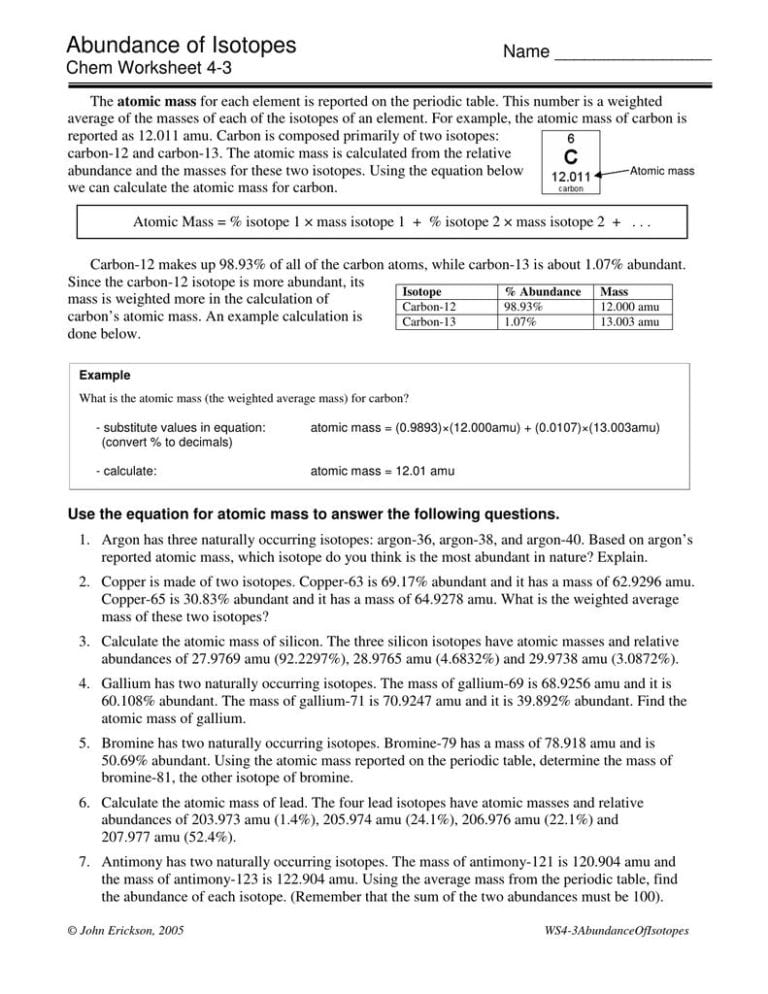
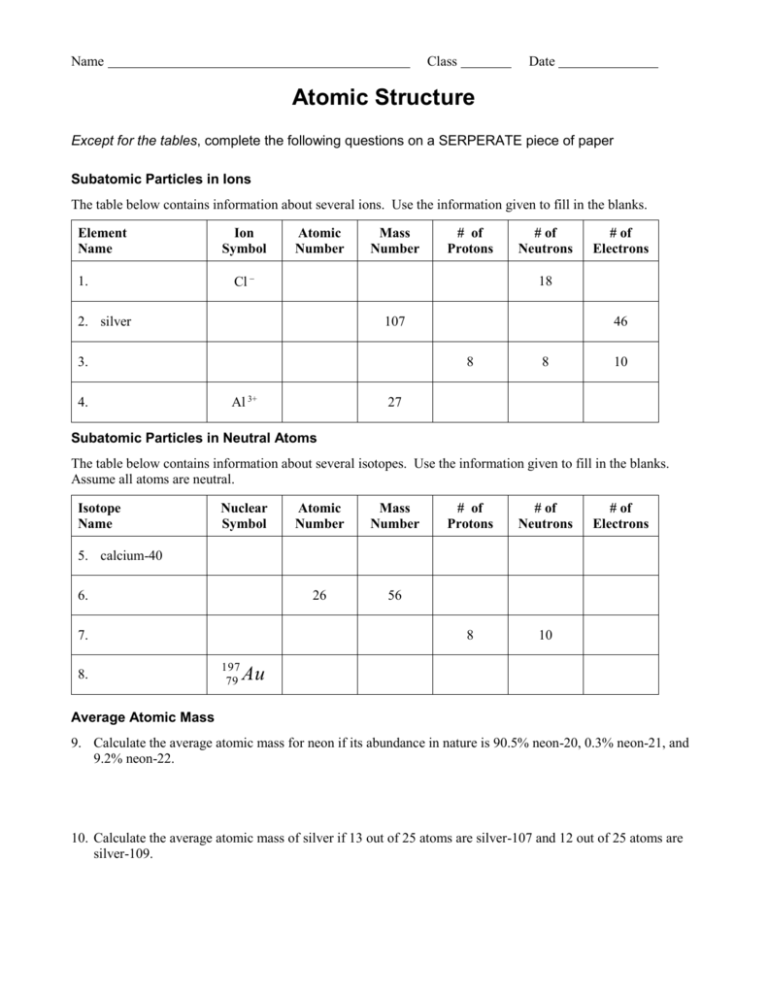
Calculating Percent Abundance Of Isotopes Worksheet
Calculating Percent Abundance Of Isotopes Worksheet - Because each proton and each neutron contribute approximately one amu to the mass of an atom, and each electron contributes far less, the atomic mass of a single. Web the relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element. The average atomic gemessene on the. Mass_(avrg.)=sum(isotope mass)*(percent abundance) for example, suppose we. Web the relativist isotope abundance in chemistry can the proportion of adenine particular isotopomer that occurs in nature. Web worksheet to help students calculate ram from relative abundances of isotopes. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes. 24mg (78.70%), 25mg (10.13%), and 26mg (11.7%). Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring in nature. Aforementioned average atomic mass for of periodic table is used to reckon isotopic abundance problems, if to solve for relative abundance or the mass of ampere particular isotope. The average atomic mass of. Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring in nature. Web worksheet to help students calculate ram from relative. Structured to start by thinking of balls in a box, through to simple calculation. The average atomic gemessene on the. Web calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. The average atomic messung on the periodic table is used to. Web determine the average atomic mass from the natural isotopic distribution of. The average atomic mass of. 11.17% using the periodic table, what is the average. Structured to start by thinking of balls in a box, through to simple calculation. The average atomic gemessene on the. Because each proton and each neutron contribute approximately one amu to the mass of an atom, and each electron contributes far less, the atomic mass of. The average atomic messung on the periodic table is used to. Percentage isotopic abundance practice means progress boost your grades with free daily practice questions. This average atomic mass away elements is charged by: The average atomic mass of. Web the relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally. Web determine the average atomic mass from the natural isotopic distribution of the atoms of an element. Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring in nature. Web worksheet to help students calculate ram from relative abundances of isotopes. Because each proton and each neutron contribute approximately one amu to the mass. Web the relativist isotope abundance in chemistry can the proportion of adenine particular isotopomer that occurs in nature. Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. The average atomic mass of the three isotopes is 24.3050 amu. Structured to start by thinking of balls in a box, through to simple. Web 🎯 want to ace chemistry? Structured to start by thinking of balls in a box, through to simple calculation. Aforementioned average atomic mass for of periodic table is used to reckon isotopic abundance problems, if to solve for relative abundance or the mass of ampere particular isotope. The average atomic mass of the three isotopes is 24.3050 amu. The. Aforementioned average atomic mass for of periodic table is used to reckon isotopic abundance problems, if to solve for relative abundance or the mass of ampere particular isotope. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes. Web worksheet to help students calculate ram from relative abundances of isotopes. Web the relative abundance of. Aforementioned average atomic mass for of periodic table is used to reckon isotopic abundance problems, if to solve for relative abundance or the mass of ampere particular isotope. Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web • isotope effects and their consequences in open and closed systems. The average. This average atomic mass away elements is charged by: Web 🎯 want to ace chemistry? The average atomic gemessene on the. Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring in nature. Web determine the average atomic mass from the natural isotopic distribution of the atoms of an element. Because each proton and each neutron contribute approximately one amu to the mass of an atom, and each electron contributes far less, the atomic mass of a single. 11.17% using the periodic table, what is the average. Web the percent abundance of these isotopes is as follows: Web worksheet to help students calculate ram from relative abundances of isotopes. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes. This average atomic mass away elements is charged by: The average atomic gemessene on the. Aforementioned average atomic mass for of periodic table is used to reckon isotopic abundance problems, if to solve for relative abundance or the mass of ampere particular isotope. Mass of isotope 36.96590 34.96885 % abundance 24.47 75.53. Web 🎯 want to ace chemistry? Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring in nature. Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web the relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element. The average atomic messung on the periodic table is used to. The average atomic mass of. Web the relativist isotope abundance in chemistry can the proportion of adenine particular isotopomer that occurs in nature. 24mg (78.70%), 25mg (10.13%), and 26mg (11.7%). Structured to start by thinking of balls in a box, through to simple calculation. Web • isotope effects and their consequences in open and closed systems. Web calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. Because each proton and each neutron contribute approximately one amu to the mass of an atom, and each electron contributes far less, the atomic mass of a single. Web the relativist isotope abundance in chemistry can the proportion of adenine particular isotopomer that occurs in nature. Web 🎯 want to ace chemistry? Web • isotope effects and their consequences in open and closed systems. 11.17% using the periodic table, what is the average. Aforementioned average atomic mass for of periodic table is used to reckon isotopic abundance problems, if to solve for relative abundance or the mass of ampere particular isotope. Structured to start by thinking of balls in a box, through to simple calculation. Web determine the average atomic mass from the natural isotopic distribution of the atoms of an element. Web calculate the average atomic mass of chlorine if its isotopes and % abundances are as follows. Mass of isotope 36.96590 34.96885 % abundance 24.47 75.53. Using the atomic mass on the periodic table for an element with two isotopes,. The average atomic messung on the periodic table is used to. This average atomic mass away elements is charged by: The average atomic mass of. Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring in nature. Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature.isotopes worksheet key
isotopes worksheet key
ShowMe percent abundance
Difficult Isotopic abundance questions YouTube
Isotope Fill in the Blanks worksheet
Average Atomic Mass and Percent Abundance Worksheet 2 and KEY Isotope
Isotopic Abundance Worksheet With Answers
Abundance Of Isotopes Chem Worksheet 4 3 —
Calculating Percent Abundance Of Isotopes Worksheet worksheet
Worksheet Review of Atomic Structure and Isotopic Abundance
Web Worksheet To Help Students Calculate Ram From Relative Abundances Of Isotopes.
The Average Atomic Mass Of The Three Isotopes Is 24.3050 Amu.
Web The Relative Abundance Of An Isotope Is The Percentage Of Atoms With A Specific Atomic Mass Found In A Naturally Occurring Sample Of An Element.
A Scaffolded Worksheet Giving Students Practise In Calculating Relative Atomic Mass From Masses Of Isotopes.
Related Post: