Calculating Ph And Poh Worksheet
Calculating Ph And Poh Worksheet - A 0.023 m solution of hydrochloric acid. Web to calculate the ph of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter (molarity). Web highlights learning objectives by the end of this section, you will be able to: If we use jay's example here at 50°c, then ph +. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web richard a year ago correct. Calculate the ph and the poh of each of the following solutions at 25 °c for which the substances ionize completely: Ph and poh calculations part 1 : 3.5 0.35 4 12 next. Web we will calculate the ph of the solutions using the following 3 steps for each problem. Explain the characterization of aqueous solutions as acidic, basic, or neutral express hydronium and. Student will also familiarizing themselves with the. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Ph and poh calculations part 1 : The ph is given by the equation. What is the ph of the solution? Web free collection of ph and poh calculation worksheets for students if you want to calculate the ph , you have to take the negative log of the hydronium ion concentration. What is left in solution? Web richard a year ago correct. Ph and poh calculations part 1 : For example the ph of a solution is given by. Web we will calculate the ph of the solutions using the following 3 steps for each problem. [oh−] = 4.59 × 10−13 m 4. What is the ph of the solution? [oh −] = 0.0044 m × 2 = 0.0088m. Web we will calculate the ph of the solutions using the following 3 steps for each problem. Choose an answer and hit 'next'. Ph and poh calculations part 1 : Web to calculate the ph of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter (molarity). Web ph = − log[h 3o. Calculate the ph by rearranging and plugging in the poh: At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. The ph is given by the equation. Ph = 14.00 − poh = 14.00 − 2.06 = 11.94. Web calculate the poh by plugging the [oh −] into the equation. Web richard a year ago correct. Ph and poh calculations part 1 : Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. Calculate the ph and the poh of each of the following solutions at 25 °c for which the substances ionize completely: A 0.0334 m solution of potassium. Web up to 6% cash back we can then derive the poh from the ph value. [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: Calculate the ph and the poh of each of the following solutions at 25 °c for which the substances ionize completely: The ph is given by the equation.. [h+] = 4.29 × 10−11 m. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. Web highlights learning objectives by the end of this section, you will be able to: Web to calculate the ph of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter (molarity). A 0.0334. Ph = 14.00 − poh = 14.00 − 2.06 = 11.94. Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. Calculate the ph and the poh of each of the following solutions at 25 °c for which the substances ionize completely: Choose an answer and hit 'next'. Student will also familiarizing themselves with. Explain the characterization of aqueous solutions as acidic, basic, or neutral express hydronium and. [oh−] = 4.59 × 10−13 m 4. Calculate the ph and the poh of each of the following solutions at 25 °c for. The ph is given by the equation. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Student will also familiarizing themselves with the. Ph = − log[h3o +] = − log(0.10) = 1.00. For example the ph of a solution is given by. [oh−] = 4.59 × 10−13 m 4. Web find the ph and the poh of the solution. [oh −] = 0.0044 m × 2 = 0.0088m. Web richard a year ago correct. Calculate the ph and the poh of each of the following solutions at 25 °c for which the substances ionize completely: Fill in the missing information in the table below. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. The ph is given by the equation. Calculate the ph by rearranging and plugging in the poh: 3.5 0.35 4 12 next. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. You will receive your score and answers at the end. Calculate the ph and the poh of each of the following solutions at 25 °c for. Ph = 14.00 − poh = 14.00 − 2.06 = 11.94. Explain the characterization of aqueous solutions as acidic, basic, or neutral express hydronium and. [h+] = 4.29 × 10−11 m. Web up to 6% cash back we can then derive the poh from the ph value. Ph = − log[h3o +] = − log(0.10) = 1.00. You will receive your score and answers at the end. Web calculate the poh by plugging the [oh −] into the equation. 3.5 0.35 4 12 next. For example the ph of a solution is given by. [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: [oh−] = 4.59 × 10−13 m 4. Poh = − log[oh −] = − log(0.0088) = 2.06. A 0.023 m solution of hydrochloric acid. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. Web richard a year ago correct. What is left in solution? The ph is then calculated using the. Calculate the ph and the poh of each of the following solutions at 25 °c for. Web free collection of ph and poh calculation worksheets for students if you want to calculate the ph , you have to take the negative log of the hydronium ion concentration. The ph is given by the equation.Calculating Ph And Poh Worksheet
Ph And Poh Worksheet Answers
Ph And Poh Worksheet Answer Key Kayra Excel
30 Ph and Poh Worksheet Education Template
Ph And Poh Calculations Worksheet
Ph And Poh Worksheet Answers
50 Ph and Poh Worksheet Chessmuseum Template Library
Calculating Ph And Poh Worksheet
Ph And Poh Calculations Worksheet —
Ph And Poh Worksheet Answers
Explain The Characterization Of Aqueous Solutions As Acidic, Basic, Or Neutral Express Hydronium And.
Poh = − Log[Oh −] = − Log(4.9 × 10 − 7) = 6.31.
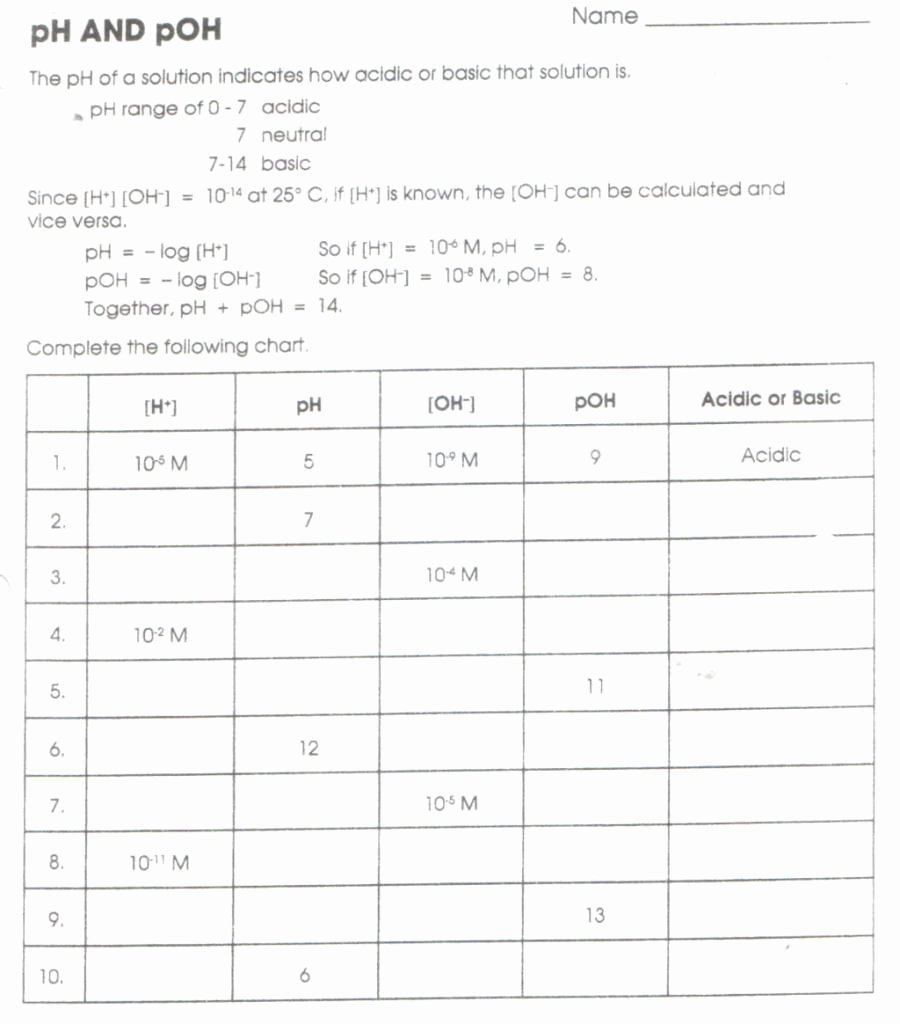
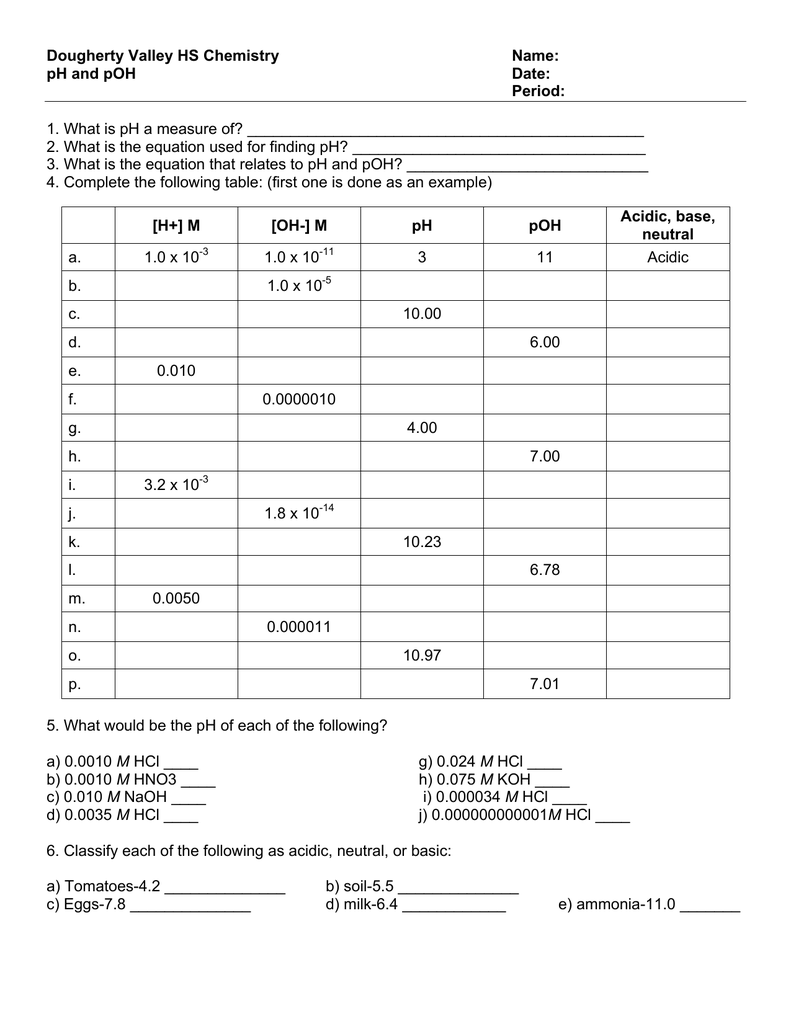
Fill In The Missing Information In The Table Below.
Web Find The Ph And The Poh Of The Solution.
Related Post:






:max_bytes(150000):strip_icc()/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)