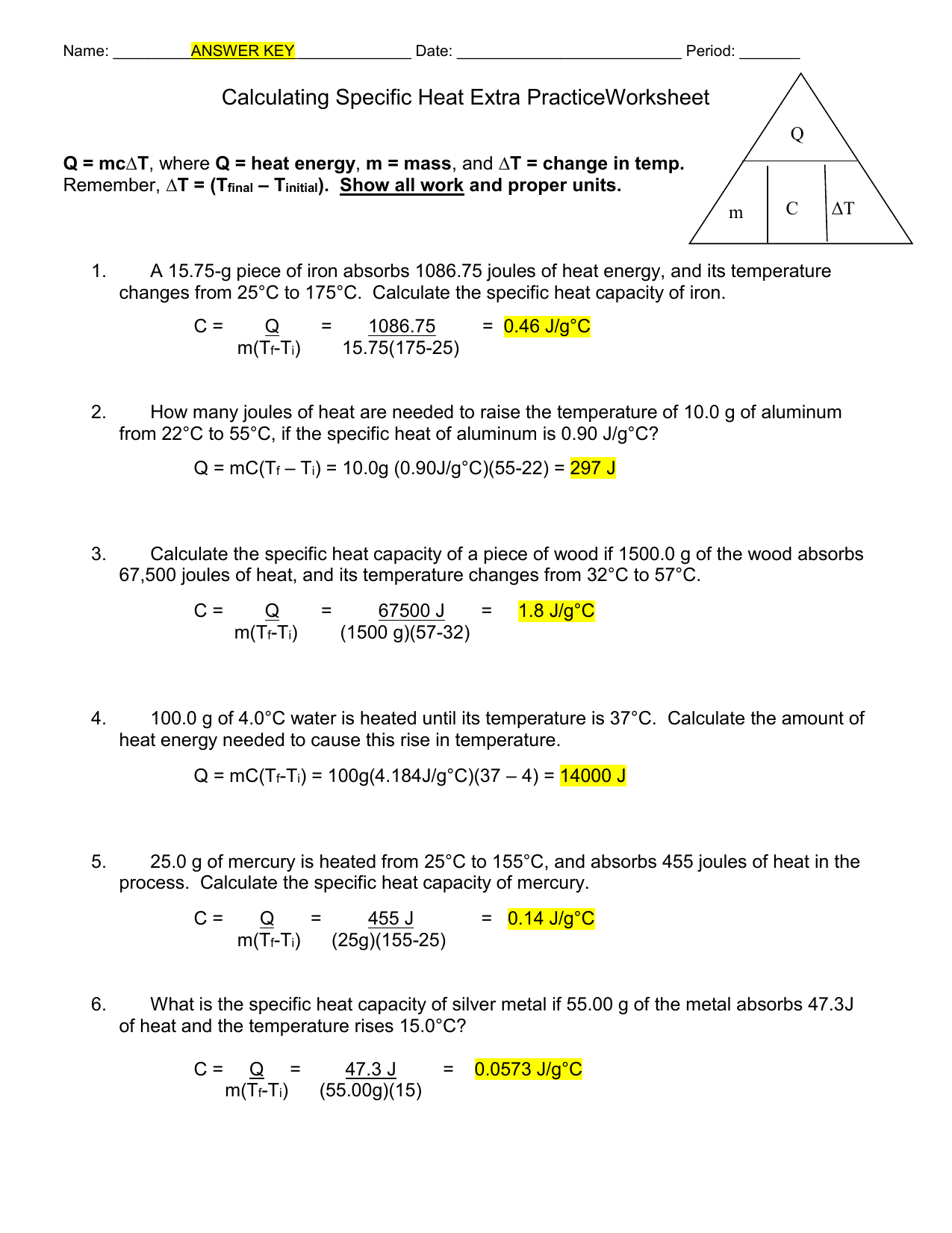
Calculating Specific Heat Worksheet Answers
Calculating Specific Heat Worksheet Answers - Web 1) solve for the heat required to increase the water temperature from 33.0 oc to 100.0 oc. Web science chemistry chemistry questions and answers calculating specific heat worksheet q = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to change from 10°c to 29°c? Web answers = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Specific heat capacity is the. Web the specific heat of the solution is generally assumed to be the same as that of pure water, 4.184 j/g × k. Web this quiz and worksheet gauge your knowledge of specific heat capacity and how it is calculated. Web particular warmth calculations answers. Use q = (m)(δt)(cp) to solve the following problems. The heat capacity of the calorimeter is calculated as. The heat capacity of the calorimeter is calculated as. Specific heat capacity is the. How much heat is absorbed by a 600 g iron cornbread pan when its temperature rises from. Stop here because the water will change phase at this temperature. You will be quizzed on terms, such as heat energy and kinetic energy. Specific heat calculations , comparing specific heats of materials, interpreting heating and cooling graphs of materials, making predictions, calculating. Heat and heat calculations name 1. Specific warmth issues 1 how much heat must be absorbed by 375 grams of water to boost its temperature by 25 c. Show all work and proper units. Energy is stored is energy in motion. How much heat is needed to raise the temperature of 50.0 g of water by 25.0°c 2. Energy is stored is energy in motion. You will be quizzed on terms, such as heat energy and kinetic energy. What is the difference in temperature and heat? Specific heat calculations , comparing specific heats of materials, interpreting heating and cooling graphs of. You will be quizzed on terms, such as heat energy and kinetic energy. Web what is the mass of the sample? What is the difference in temperature and heat? Web this quiz and worksheet gauge your knowledge of specific heat capacity and how it is calculated. Energy is stored is energy in motion. Web let's take a look at how we can use the specific heat equation to calculate the final temperature: Stop here because the water will change phase at this temperature. Specific heat capacity is the. Use q = (m)(δt)(cp) to solve the following problems. What is the difference in temperature and heat? Web let's take a look at how we can use the specific heat equation to calculate the final temperature: Web this quiz and worksheet gauge your knowledge of specific heat capacity and how it is calculated. How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to change from 10°c to 29°c? Web. You will be quizzed on terms, such as heat energy and kinetic energy. What is the difference in temperature and heat? Web particular warmth calculations answers. Web the specific heat of the solution is generally assumed to be the same as that of pure water, 4.184 j/g × k. You will receive your score and answers at the end. Web answers = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Energy is stored is energy in motion. How much heat is needed to raise the temperature of 50.0 g of water by 25.0°c 2. How much heat is absorbed by a 600 g iron cornbread pan when its temperature rises from. Specific. How much heat is needed to raise the temperature of 50.0 g of water by 25.0°c 2. How much heat is absorbed by a 600 g iron cornbread pan when its temperature rises from. The heat capacity of the calorimeter is calculated as. 2) solve for the heat. What is the difference in temperature and heat? Web excellent worksheet that helped me practice the specific heat capacity equation in preparation for my mock exam. Specific heat capacity is the. Web this quiz and worksheet gauge your knowledge of specific heat capacity and how it is calculated. Web particular warmth calculations answers. The amount of heat required to raise the temperature of one gram of a substance. Web the symbol c stands for specific heat, and depends on the material and phase. The amount of heat required to raise the temperature of one gram of a substance by one celsius degree is referred to. Energy is stored is energy in motion. How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to change from 10°c to 29°c? 2) solve for the heat. Show all work and units. Web excellent worksheet that helped me practice the specific heat capacity equation in preparation for my mock exam. How much heat is needed to raise the temperature of 50.0 g of water by 25.0°c 2. Specific heat capacity is the. How much heat is absorbed by a 600 g iron cornbread pan when its temperature rises from. The heat capacity of the calorimeter is calculated as. Example 2 what is the final temperature if 100.0 j is added to 10.0 g of. Web answers = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. You will receive your score and answers at the end. The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of. What is the difference in temperature and heat? Heat and heat calculations name 1. Web choose an answer and hit 'next'. Specific warmth issues 1 how much heat must be absorbed by 375 grams of water to boost its temperature by 25 c. In this lesson we looked at two different formulas for heat added to a. Web excellent worksheet that helped me practice the specific heat capacity equation in preparation for my mock exam. Energy is stored is energy in motion. 2) solve for the heat. Web the symbol c stands for specific heat, and depends on the material and phase. Web what is the mass of the sample? Example 2 what is the final temperature if 100.0 j is added to 10.0 g of. Web particular warmth calculations answers. In this lesson we looked at two different formulas for heat added to a. When you heat a substance and the temperature rises, how much it rises depends on its ______ ______ _________. How much heat is needed to raise the temperature of 50.0 g of water by 25.0°c 2. The amount of heat required to raise the temperature of one gram of a substance by one celsius degree is referred to. Show all work and proper units. Web answers = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. The specific heat is the amount of heat necessary to change the temperature of 1.00 kg of. Web this quiz and worksheet gauge your knowledge of specific heat capacity and how it is calculated. Specific heat calculations , comparing specific heats of materials, interpreting heating and cooling graphs of materials, making predictions, calculating.Calculating Specific Heat Worksheet Answers
30 Specific Heat Worksheet Answer Key Education Template
Specific Heat Worksheet Answers
Calculating Specific Heat Extra Practice Worksheet
Specific Heat Worksheet Answers
Specific Heat Worksheet Answers
Specific Heat Worksheet Answers
Calculating Specific Heat Worksheet Answers
Calculating Specific Heat Worksheet Answers Yooob —
Specific Heat Worksheet Answer Key
Web Specific Heat Worksheet Specific Heat Directions:
The Heat Capacity Of The Calorimeter Is Calculated As.
How Much Heat Is Absorbed By 20G Granite Boulder As Energy From The Sun Causes Its Temperature To Change From 10°C To 29°C?
Web Choose An Answer And Hit 'Next'.
Related Post: