Calorimetry Problems Worksheet
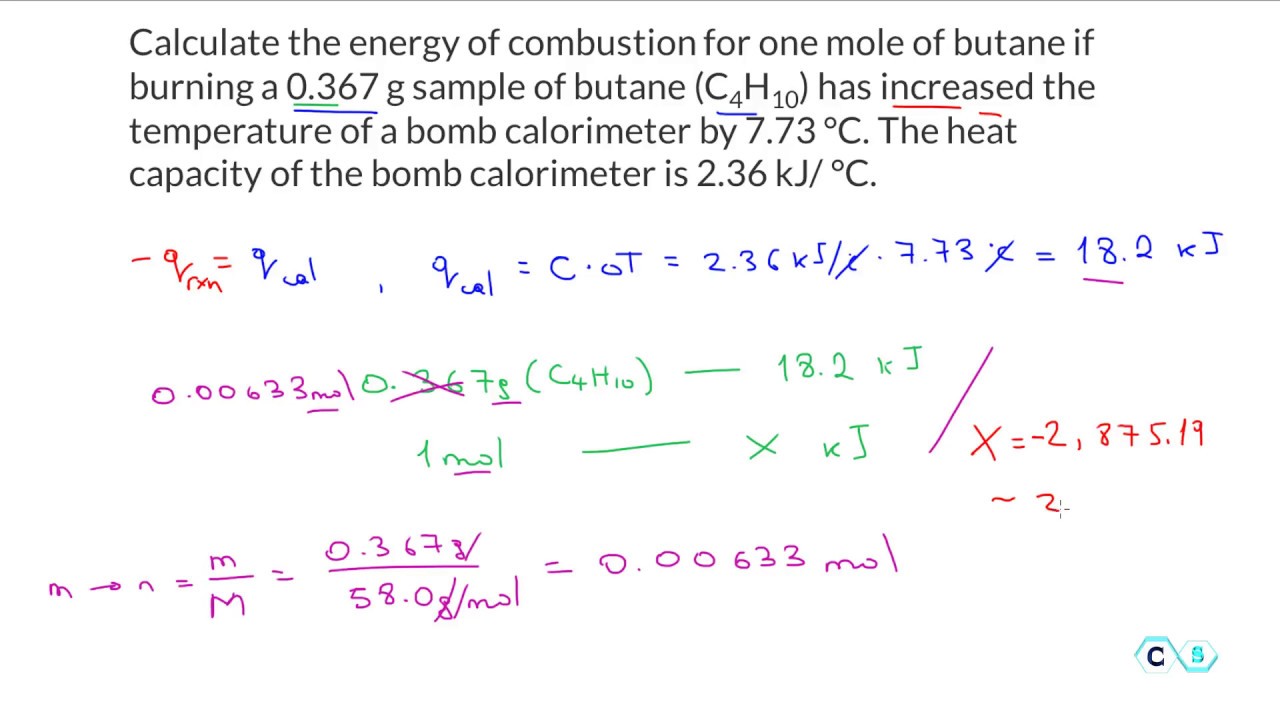
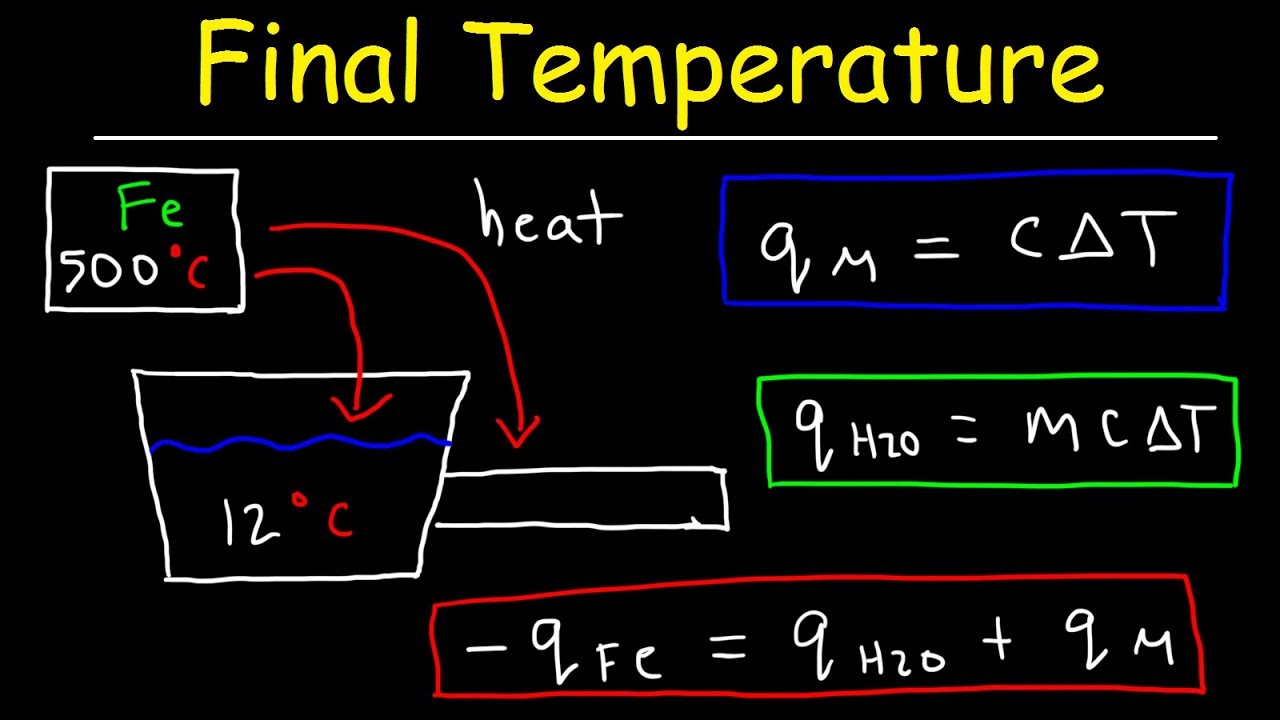
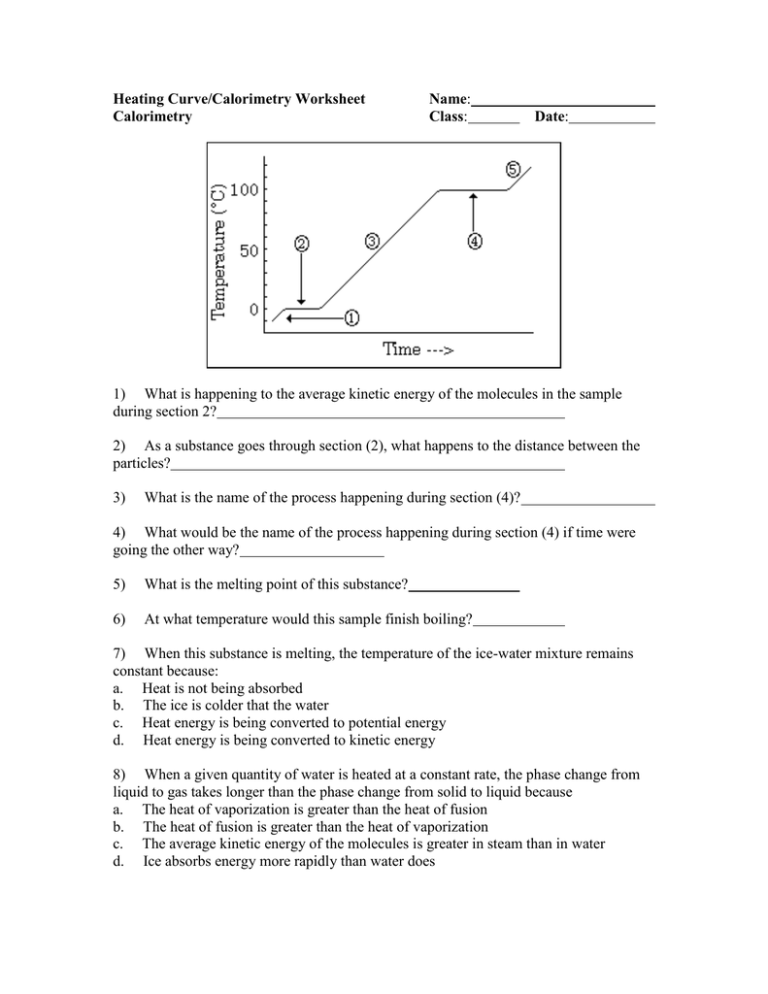
Calorimetry Problems Worksheet - Web calorimetry is a complicated science. What temperature change will 100.0 ml of water. Exactly 25 000 j of heat is added to a calorimeter containing 100.0 g of water at an final temperature of 88.0. This quiz/worksheet will help you assess your understanding of how to calculate temperature and heat capacity and let you put your. Understand the relationships between heat, work, internal energy, and enthalpy. Worksheets are calorimetry work, calorimetry work w 337, calorimetry, calorimetry work, work calorimetry. Web know the first law of thermodynamics. Web how much heat is released when 275 grams of water cools from 85.2 degrees celsius to 28.4 degrees celsius? Web showing 8 worksheets for calorimetry practice problem. Web calorimetry worksheet w 337 everett community college tutoring center student support services program c p (h 2 o) = 4.184 j / g 0c h = mc p t 1) a compound is. Exactly 25 000 j of heat is added to a calorimeter containing 100.0 g of water at an final temperature of 88.0. Web how much heat is released when 275 grams of water cools from 85.2 degrees celsius to 28.4 degrees celsius? Worksheets are calorimetry work w 337, ii calorimetry work, chemistry work heat and calorimetry. The mass of a. Web assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat absorbed by the reaction, which can be represented by the. Web figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its. When. Web up to 24% cash back 6. This assignment gives students practice performing enthalpy calorimetry calculations. Exactly 25 000 j of heat is added to a calorimeter containing 100.0 g of water at an final temperature of 88.0. Web know the first law of thermodynamics. A sample of water with a mass of 21.0 grams is cooled from 44.7 oc. Exactly 25 000 j of heat is added to a calorimeter containing 100.0 g of water at an final temperature of 88.0. This assignment gives students practice performing enthalpy calorimetry calculations. Worksheets are calorimetry work w 337, ii calorimetry work, chemistry work heat and calorimetry. Web assuming the specific heat of the solution and products is 4.20 j/g °c, calculate. Web figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its. Web this is a power point on calorimetry, using the equation q = mct to solve problems, calorimeters, specific heat capacity, enthalpy of physical changes and calorimetry. Web how much heat. Worksheets are calorimetry work w 337, ii calorimetry work, chemistry work heat and calorimetry. Web assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat absorbed by the reaction, which can be represented by the. Web up to 24% cash back 6. Web calorimetry is a complicated science. Identify the mass. Specific heat capacityreal examples of specific heatcalorimetry (including parts of a. Web figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its. Web showing 8 worksheets for calorimetry practice problem. How many joules of heat were lost in the cooling process? Exactly. Exactly 25 000 j of heat is added to a calorimeter containing 100.0 g of water at an final temperature of 88.0. Web this is a power point on calorimetry, using the equation q = mct to solve problems, calorimeters, specific heat capacity, enthalpy of physical changes and calorimetry. Web showing 8 worksheets for calorimetry practice problem. Identify the mass. Web figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its. Web calorimetry practice problems 1. A sample of water with a mass of 21.0 grams is cooled from 44.7 oc to 28.1 oc. Web sch4u thermodynamics & calorimetry energy changes &. Specific heat capacityreal examples of specific heatcalorimetry (including parts of a. When 5.000 grams of ammonia react with an excess of oxygen and ch 4 in a bomb calorimeter with a total heat capacity of 15.48 kj/°c, the. Identify the mass of the substance and the specific heat capacity constant for the substance. Web up to 24% cash back 6.. Web calorimetry worksheet w 337 everett community college tutoring center student support services program c p (h 2 o) = 4.184 j / g 0c h = mc p t 1) a compound is. How many joules of heat were lost in the cooling process? Web sch4u thermodynamics & calorimetry energy changes & rates of reactions first law of thermodynamics: Understand the concepts of heat. Exactly 25 000 j of heat is added to a calorimeter containing 100.0 g of water at an final temperature of 88.0. Identify the mass of the substance and the specific heat capacity constant for the substance. What temperature change will 100.0 ml of water. Web assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat absorbed by the reaction, which can be represented by the. Web this worksheet set guides students through the following topics: The mass of a piece. Web how much heat is released when 275 grams of water cools from 85.2 degrees celsius to 28.4 degrees celsius? This assignment gives students practice performing enthalpy calorimetry calculations. Web calorimetry practice problems 1. Determine the initial temperature of the water. Specific heat capacityreal examples of specific heatcalorimetry (including parts of a. Identify the change in temperature by t = t f i n a l − t i n i t i a l step. This assignment gives students practice performing enthalpy calorimetry calculations. This quiz/worksheet will help you assess your understanding of how to calculate temperature and heat capacity and let you put your. Web this is a power point on calorimetry, using the equation q = mct to solve problems, calorimeters, specific heat capacity, enthalpy of physical changes and calorimetry. A sample of water with a mass of 21.0 grams is cooled from 44.7 oc to 28.1 oc. Web up to 24% cash back 6. Understand the concepts of heat. Web this worksheet set guides students through the following topics: Exactly 25 000 j of heat is added to a calorimeter containing 100.0 g of water at an final temperature of 88.0. A sample of water with a mass of 21.0 grams is cooled from 44.7 oc to 28.1 oc. Specific heat capacityreal examples of specific heatcalorimetry (including parts of a. Web how much heat is released when 275 grams of water cools from 85.2 degrees celsius to 28.4 degrees celsius? What temperature change will 100.0 ml of water. Web figure 5.11 in a calorimetric determination, either (a) an exothermic process occurs and heat, q, is negative, indicating that thermal energy is transferred from the system to its. Web showing 8 worksheets for calorimetry practice problem. Web assuming the specific heat of the solution and products is 4.20 j/g °c, calculate the approximate amount of heat absorbed by the reaction, which can be represented by the. Web calorimetry practice problems 1. Web calorimetry is a complicated science. Web calorimetry worksheet w 337 everett community college tutoring center student support services program c p (h 2 o) = 4.184 j / g 0c h = mc p t 1) a compound is. This quiz/worksheet will help you assess your understanding of how to calculate temperature and heat capacity and let you put your. When 5.000 grams of ammonia react with an excess of oxygen and ch 4 in a bomb calorimeter with a total heat capacity of 15.48 kj/°c, the.Calorimetry Problems Worksheet Answers Go Images Road
Chemistry Worksheet Heat And Calorimetry Problems Answers Chemistry
30 Calorimetry Worksheet Answer Key Education Template
Calorimetry Practice Worksheet Answers Escolagersonalvesgui
CALORIMETRY WORKSHEET 2
Calorimetry Problems 1
calorimetry worksheet 1 answers
Calorimetry practice
30 Calorimetry Worksheet Answer Key Education Template
Calorimetry Worksheet Answer Key
This Assignment Gives Students Practice Performing Enthalpy Calorimetry Calculations.
Identify The Change In Temperature By T = T F I N A L − T I N I T I A L Step.
Web This Is A Power Point On Calorimetry, Using The Equation Q = Mct To Solve Problems, Calorimeters, Specific Heat Capacity, Enthalpy Of Physical Changes And Calorimetry.
The Mass Of A Piece.
Related Post: