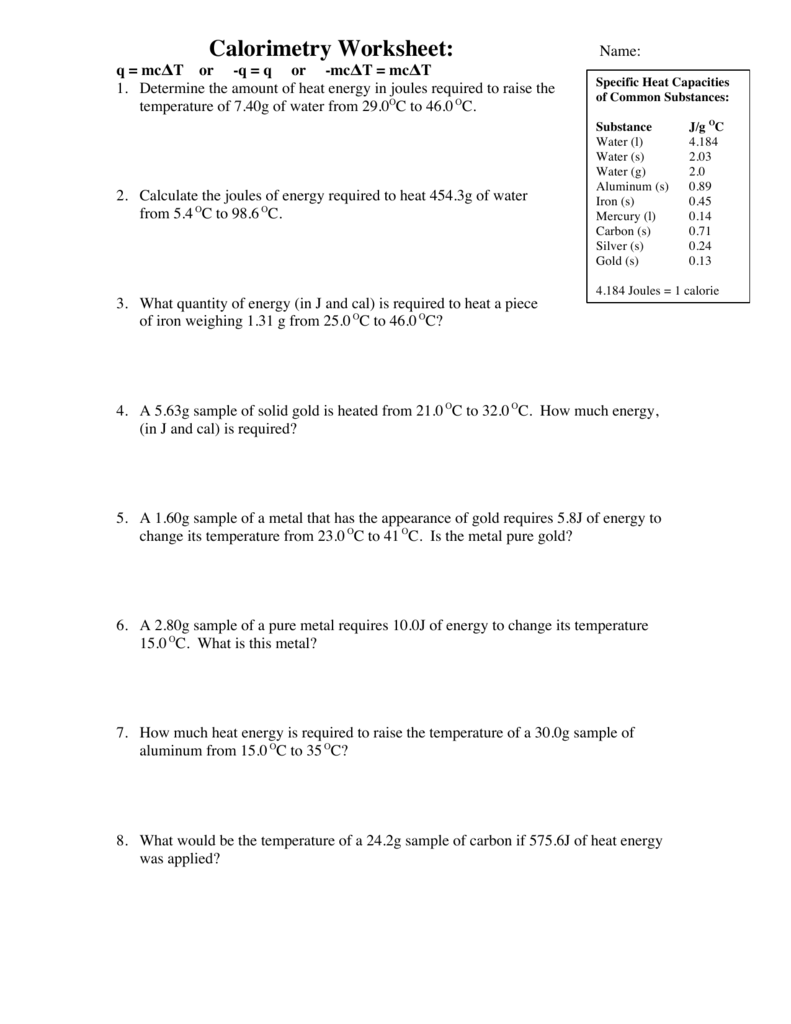
Calorimetry Worksheet Answers
Calorimetry Worksheet Answers - Web calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate the energy, in calories, for 15 pretzels if the raise the temperature of 150 g of water in a calorimeter by 45.3oc. Chapter 6 calorimetry worksheet key open the calorimetry lab activity (section 6.6, figure 6.11) to complete this worksheet. The specific heat of iron (at. What is the value of the molar heat capacity of iron? = 55.85 u) is 0.450 j/g@k. Question 1 of 3 express 55.3 j of. Web calorimetry heat absorbed or liberated by a chemical reaction can be determined with a calorimeter. T f = 28.58 0c b. Web please answer the following questions and show all work. If the combustion of 0.175 moles of this compound causes the temperature of. Place 200 ml of room temperature water from a carboy in a 250 ml beaker and set it aside for later use. A sheet of paper, marked with a. Web in this set of practice questions, we will go over the main types of questions on calorimetry. Web calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. Choose an answer and hit 'next'. You will receive your score and answers at the end. Web this experiment is done in a team of two. The heat of the reaction is not directly observed, but. You will receive your score and answers at the end. T f = 32.86 0c (2) 25.8 kj/mol (3) 28.74 0c (4) a. Web calorimetry is used to measure amounts of heat transferred to or from a substance. The final temperature is closer to t_1 t 1 than to t_2 t 2. Web calorimetry is based on the first law. The heat of the reaction is not directly observed, but. Web calorimetry worksheet answer key. T f = 32.86 0c (2) 25.8 kj/mol (3) 28.74 0c (4) a. Web please answer the following questions and show all work. The heat of neutralization that is lost in the chemical reaction (the. Web chemistry questions and answers; To do so, the heat is exchanged with a calibrated object (calorimeter). The final temperature is closer to t_1 t 1 than to t_2 t 2. Place 200 ml of room temperature water from a carboy in a 250 ml beaker and set it aside for later use. Worksheet on calorimetry (1) a. The heat of neutralization that is lost in the chemical reaction (the. Choose an answer and hit 'next'. Place 200 ml of room temperature water from a carboy in a 250 ml beaker and set it aside for later use. Web showing 8 worksheets for calorimetry. Worksheet on calorimetry (1) a. You will receive your score and answers at the end. Web calorimetry heat absorbed or liberated by a chemical reaction can be determined with a calorimeter. Pb(no 3) 2 + 2nai 2nano 3 + pbi 2 b. Choose an answer and hit 'next'. The specific heat of iron (at. To do so, the heat is exchanged with a calibrated object (calorimeter). In a constant volume apparatus like a calorimeter, the change in internal. Web specific heat and calorimetry (heat lost=heat gained) worksheet. Web showing 8 worksheets for calorimetry. T f = 28.58 0c b. In a constant volume apparatus like a calorimeter, the change in internal. How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? Web calorimetry heat absorbed or liberated by a chemical reaction can be determined with a calorimeter. Question 1 of 3 express 55.3 j of. Web specific heat and calorimetry (heat lost=heat. Web another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by 1.00 o c. Web this experiment is done in a team of two. In a constant volume apparatus like a calorimeter, the change in internal. T f = 32.86 0c (2). How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? Web in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the final temperature of a. Worksheet on calorimetry (1) a. The specific heat of iron (at. = (0.450 j/g@k)(23.5 g) = 10.5 75 j/k = 10.6 j/k = (0.450 j/g@k)(55.85 g/mol) = 25.1 j/mol@k = 25.1 m325j/mol@k 6. Worksheets are calorimetry work, calorimetry work w 337, calorimetry, calorimetry work, work calorimetry calorim. Web calorimetry is based on the first law of thermodynamics that states that energy cannot be created nor destroyed. The final temperature is exactly halfway. Choose an answer and hit 'next'. Web calorimetry is used to measure amounts of heat transferred to or from a substance. Web up to 24% cash back 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Web chemistry questions and answers; Question 1 of 3 express 55.3 j of. To do so, the heat is exchanged with a calibrated object (calorimeter). Web calorimetry worksheet answer key. Pb(no 3) 2 + 2nai 2nano 3 + pbi 2 b. Place 200 ml of room temperature water from a carboy in a 250 ml beaker and set it aside for later use. Web showing 8 worksheets for calorimetry. A sheet of paper, marked with a. The heat of the reaction is not directly observed, but. = 55.85 u) is 0.450 j/g@k. Web this experiment is done in a team of two. The heat of neutralization that is lost in the chemical reaction (the. You will receive your score and answers at the end. Web chemistry questions and answers; The heat of the reaction is not directly observed, but. = (0.450 j/g@k)(23.5 g) = 10.5 75 j/k = 10.6 j/k = (0.450 j/g@k)(55.85 g/mol) = 25.1 j/mol@k = 25.1 m325j/mol@k 6. Question 1 of 3 express 55.3 j of. Chapter 6 calorimetry worksheet key open the calorimetry lab activity (section 6.6, figure 6.11) to complete this worksheet. Web another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by 1.00 o c. What is the value of the molar heat capacity of iron? Worksheets are calorimetry work, calorimetry work w 337, calorimetry, calorimetry work, work calorimetry calorim. Web showing 8 worksheets for calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). The specific heat of iron (at. The final temperature is closer to t_1 t 1 than to t_2 t 2.Awasome Calorimetry Worksheet Answer Key 2023 Alec Worksheet
Calorimetry Worksheet Answer Key
Calorimetry Lab Gizmo Answers Activity C / Calorimetry Lab Gizmo
Calorimetry Worksheet Answer Key
Creatively
Heat Capacity Calorimetry Worksheet answers 1151 Chemistry OFTC
Calorimetry Worksheet Answer Key
Calorimetry Worksheet Answer Key
30 Calorimetry Worksheet Answer Key Education Template
30 Calorimetry Worksheet Answer Key Education Template
What Are The Two Main Ways In Which The Internal Vitality Of An Object Could Be Categorized?
T F = 28.58 0C B.
Pb(No 3) 2 + 2Nai 2Nano 3 + Pbi 2 B.
The Final Temperature Is Closer To T_1 T 1 Than To T_2 T 2.
Related Post: