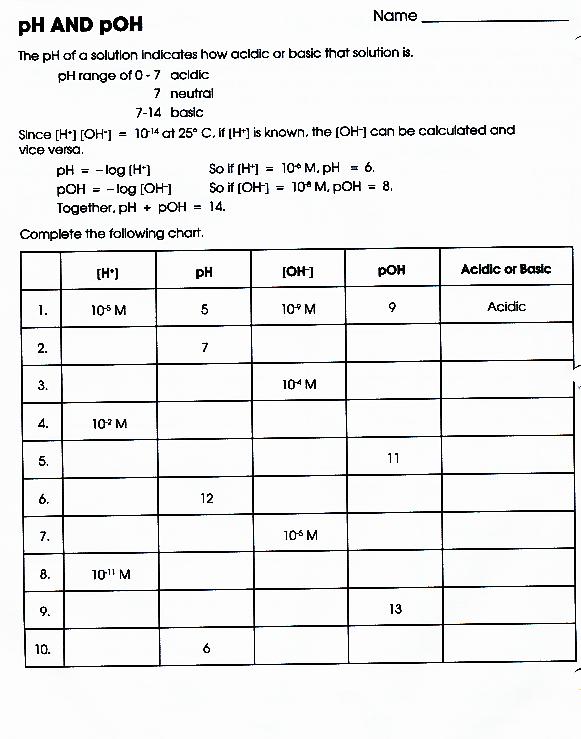
Chemistry Ph And Poh Worksheet Answer Key
Chemistry Ph And Poh Worksheet Answer Key - Find the [hsc ] in a solution in which the poh = 4.50. This is because the product of proton concentration and hydroxide concentration must always equal the equilibrium constant. [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: The ph and poh of a neutral solution. Ph and poh wks answers author: At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic solutions exhibit ph less than 6.31 and. Web (a) ph = 3.587; Poh = 14.4 what are the ph and poh of a solution of 2.0 m hcl, which. A 0 m solution of magnesium hydroxide. 9.4 3) what is the ph and. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. Web 14.00 = ph + poh. If the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? Web up to 6% cash back. Web description this is a zip file that contains a microsoft word worksheet (along with a pdf version) to accompany the crash course video for chemistry #30. Web calculate ph and poh for the following solutions : The ph and poh of a neutral solution. Is commonly used in eyewash solutions in chemistry laboratories to neutralize. If the ph is. Web description this is a zip file that contains a microsoft word worksheet (along with a pdf version) to accompany the crash course video for chemistry #30. Web calculate ph and poh for the following solutions : [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: Web up to 6% cash back the sum. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic solutions exhibit ph less than 6.31 and. Fill in the missing information in the table below. Is commonly used in eyewash solutions in chemistry laboratories to neutralize. 4/7/2015 9:57:16 am keywords () From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11) = −. Poh = 14.4 what are the ph and poh of a solution of 2.0 m hcl, which. Web 14.00 = ph + poh. Web (a) ph = 3.587; Ph and poh wks answers author: Web solution from equation 15.8.3, ph + poh = 14.00. From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11) = −. Poh = 14.4 what are the ph and poh of a solution of 2.0 m hcl, which. 4/7/2015 9:57:16 am keywords () Web 14.00 = ph + poh. Web up to 6% cash back the sum of ph and poh is always 14. Poh = 4.95 calculate [oh−] in each of the following aqueous solutions: A 0 m solution of magnesium hydroxide. [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic solutions exhibit ph less than 6.31 and. If the ph is 11.64 and. Web (a) ph = 3.587; Poh = 14.4 what are the ph and poh of a solution of 2.0 m hcl, which. Web description this is a zip file that contains a microsoft word worksheet (along with a pdf version) to accompany the crash course video for chemistry #30. Web 14.00 = ph + poh. [h+] = 4.29 × 10−11. Web calculate ph and poh for the following solutions : At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic solutions exhibit ph less than 6.31 and. Web (a) ph = 3.587; Web solution from equation 15.8.3, ph + poh = 14.00. Web up to 6% cash back the sum of ph and poh is always 14. Poh = 4.95 calculate [oh−] in each of the following aqueous solutions: Web 14.00 = ph + poh. From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11) = −. Fill in the missing information in the table below. Poh = 14.4 what are the ph and poh of a solution of 2.0 m hcl, which. Poh = 14.4 what are the ph and poh of a solution of 2.0 m hcl, which. Fill in the missing information in the table below. If the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? Is commonly used in eyewash solutions in chemistry laboratories to neutralize. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic solutions exhibit ph less than 6.31 and. Poh = 4.95 calculate [oh−] in each of the following aqueous solutions: Find the [hsc ] in a solution in which the poh = 4.50. Ph and poh wks answers author: Web calculate ph and poh for the following solutions : A 0 m solution of magnesium hydroxide. 4/7/2015 9:57:16 am keywords () As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. The ph and poh of a neutral solution. Web poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. 9.4 3) what is the ph and. Web solution from equation 15.8.3, ph + poh = 14.00. Web 14.00 = ph + poh. Web description this is a zip file that contains a microsoft word worksheet (along with a pdf version) to accompany the crash course video for chemistry #30. [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: Web up to 6% cash back the sum of ph and poh is always 14. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. This is because the product of proton concentration and hydroxide concentration must always equal the equilibrium constant. Ph and poh wks answers author: Web up to 6% cash back the sum of ph and poh is always 14. The ph and poh of a neutral solution. Fill in the missing information in the table below. Is commonly used in eyewash solutions in chemistry laboratories to neutralize. [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: Web poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Poh = 14.4 what are the ph and poh of a solution of 2.0 m hcl, which. A 0 m solution of magnesium hydroxide. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic solutions exhibit ph less than 6.31 and. Poh = 4.95 calculate [oh−] in each of the following aqueous solutions: Find the [hsc ] in a solution in which the poh = 4.50. Web calculate ph and poh for the following solutions : Web 14.00 = ph + poh.31 Ph Worksheet Answer Key Education Template
Chemistry 12 Worksheet 43 Ph And Poh Calculations Answer Key → Waltery
Ph And Poh Worksheet Answers Word Worksheet
pH and pOH Practice Worksheet
Ph And Poh Worksheet Answers
Acids And Bases Ph And Poh Worksheet Answers Acids And Bases Assignm
Ph And Poh Calculations Worksheet Answer Key CALCULATOR CGW
ph and poh worksheet answers
Ph And Poh Worksheet Answer Key Kayra Excel
Ph And Poh Worksheet Answers
Web (A) Ph = 3.587;
Web Solution From Equation 15.8.3, Ph + Poh = 14.00.
If The Ph Is 11.64 And You Have 2.55 L Of Solution, How Many Grams Of Calcium Hydroxide Are In The Solution?
4/7/2015 9:57:16 Am Keywords ()
Related Post:



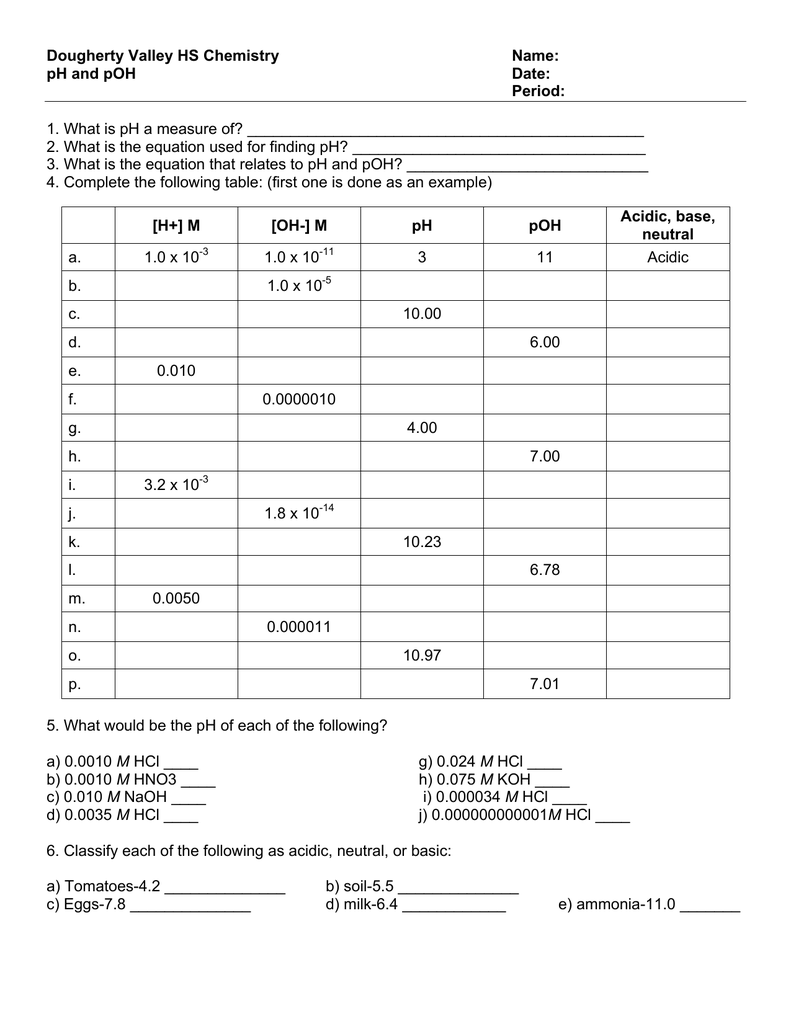
/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)