Dilution Problems Worksheet M1V1 M2V2 Answer Key
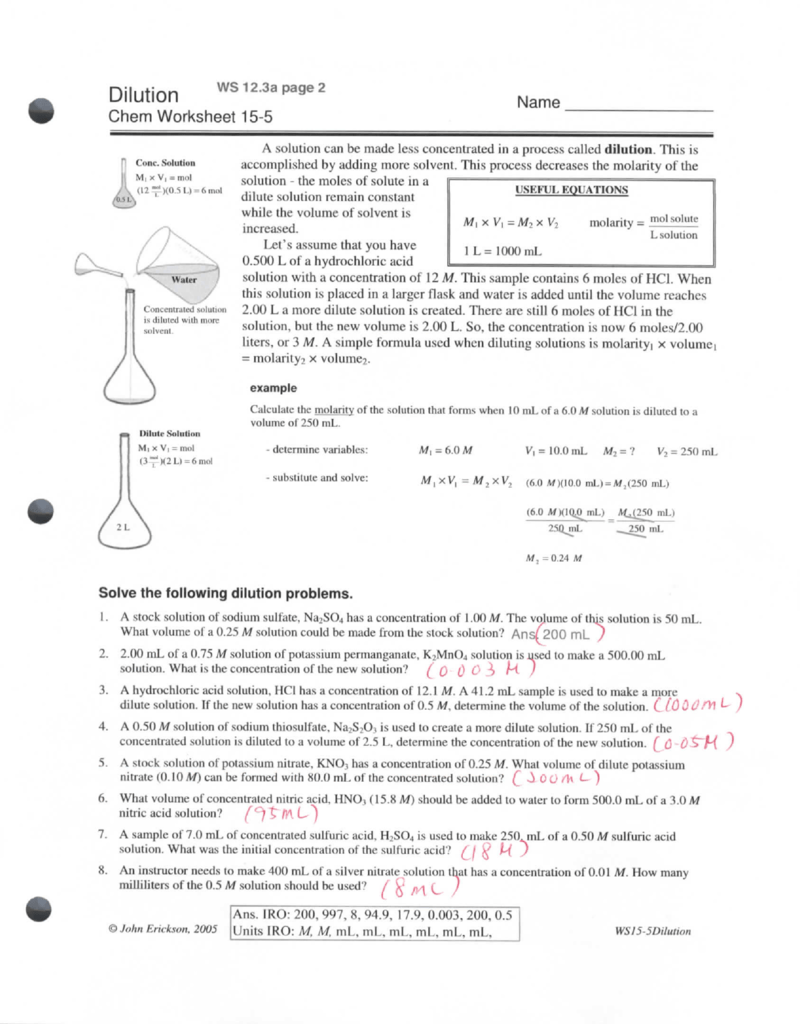
Dilution Problems Worksheet M1V1 M2V2 Answer Key - Molarity = % % % %, since we have the molarity and the volume of the. Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters. Web web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions problems using m1v1 =. Web since you will know both the moles of sugar and the volume of your solution, you will be able to calculate the molarity of the concentrated sugar sample. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Web 250.0 ml of 3.55 m hcl? Web for example if you have 5ml of a 2m solution which is diluted to a new volume of 10ml the molarity will be reduced to 1m. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Dilutions worksheet 1 if i add 25 ml of water to 125 ml of a 0 15 m naoh solution what will the molarity of the diluted solution be. Web the mole worksheet answers from printablelibrarylucas.z13.web.core.windows.net. Web 250.0 ml of 3.55 m hcl? If 455 ml of 6.0 m hno3 is. M1v1 + m2v2 = m3v3 (3.55) (0.250) + (5.65) (x) = (4.50) (0.250 + x) where x is volume of 5.65 m hcl. To solve a problem like this one you'll. How much of a 15.0 m stock solution do you need to prepare 250. Web dilutions worksheet answer key. Making dilutions worksheet m.vi = mov2 remember that you can change the concentration of a solution by adding more solvent. Web while you cannot increase the concentration of a solution in this manner, you can create a more dilute solution by increasing the amount of solvent. Units should remain constant on both sides of the. Web the mole worksheet answers from printablelibrarylucas.z13.web.core.windows.net. Web dilutions worksheet answer key. Web question dilution problems worksheet (m1v1 = m2v2) 1. Making dilutions worksheet m.vi = mov2 remember that you can change the concentration of a solution by adding more solvent. Molarity = % % % %, since we have the molarity and the volume of the. To solve a problem like this one you'll. M1v1 + m2v2 = m3v3 (3.55) (0.250) + (5.65) (x) = (4.50) (0.250 + x) where x is volume of 5.65 m hcl. Web answer key if 50.0 ml of a 1.75 m solution is diluted to 150 ml, what is the molarity of the final solution? If 455 ml of 6.0. Web answer key if 50.0 ml of a 1.75 m solution is diluted to 150 ml, what is the molarity of the final solution? Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters. Making dilutions worksheet m.vi = mov2 remember that you can change the concentration of a solution by adding. Web dilutions worksheet answer key. Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions problems using m1v1 = m2v2. Web while you cannot increase the concentration of a solution in this manner, you can create a more dilute solution by increasing the amount of solvent. Dilutions worksheet 1. Web web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions problems using m1v1=m2v2. Web we can relate the concentrations and volumes before and after a dilution using the following equation:. Web for example if you have 5ml of a 2m solution which is diluted to a new volume. Web 250.0 ml of 3.55 m hcl? Web since you will know both the moles of sugar and the volume of your solution, you will be able to calculate the molarity of the concentrated sugar sample. You can determine the amount. Web dilution problems worksheet m1v1 m2v2 answer key. How much of a 15.0 m stock solution do you need. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? You can determine the amount. This is the “m1” value in the. Web web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions problems using m1v1 =. Web. Vi ml 0 54 3qo. Units should remain constant on both sides of the equation. If 455 ml of 6.0 m. Web concentration of one solution is equal to the molarity times volume of the other solution (m₁v₁ = m₂v₂). Web dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Web we can relate the concentrations and volumes before and after a dilution using the following equation:. Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to. Here is the first way to solve this problem: M1v1 + m2v2 = m3v3 (3.55) (0.250) + (5.65) (x) = (4.50) (0.250 + x) where x is volume of 5.65 m hcl. Web the mole worksheet answers from printablelibrarylucas.z13.web.core.windows.net. Web dilutions worksheet answer key. If 455 ml of 6.0 m. Web dilution problems worksheet m1v1 m2v2 answer key. Molarity = % % % %, since we have the molarity and the volume of the. Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters. This is the “m1” value in the. Web since you will know both the moles of sugar and the volume of your solution, you will be able to calculate the molarity of the concentrated sugar sample. Dilutions worksheet 1 if i add 25 ml of water to 125 ml of a 0 15 m naoh solution what will the molarity of the diluted solution be. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Units should remain constant on both sides of the equation. Web dilution problems worksheet m1v1 m2v2 answer key. Web while you cannot increase the concentration of a solution in this manner, you can create a more dilute solution by increasing the amount of solvent. Web 250.0 ml of 3.55 m hcl? Vi ml 0 54 3qo. You can determine the amount. Making dilutions worksheet m.vi = mov2 remember that you can change the concentration of a solution by adding more solvent. This is the “m1” value in the. To solve a problem like this one you'll. Web we can relate the concentrations and volumes before and after a dilution using the following equation:. Web (53 kb | 4 pages) product description this worksheet features 5 molarity problems ( m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions. Web since you will know both the moles of sugar and the volume of your solution, you will be able to calculate the molarity of the concentrated sugar sample. Web 250.0 ml of 3.55 m hcl? M1v1 + m2v2 = m3v3 (3.55) (0.250) + (5.65) (x) = (4.50) (0.250 + x) where x is volume of 5.65 m hcl. Web dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Web web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions problems using m1v1=m2v2. Web answer key if 50.0 ml of a 1.75 m solution is diluted to 150 ml, what is the molarity of the final solution? Web while you cannot increase the concentration of a solution in this manner, you can create a more dilute solution by increasing the amount of solvent. Here is the first way to solve this problem: Molarity = % % % %, since we have the molarity and the volume of the. Dilutions worksheet 1 if i add 25 ml of water to 125 ml of a 0 15 m naoh solution what will the molarity of the diluted solution be. Web the mole worksheet answers from printablelibrarylucas.z13.web.core.windows.net.Solutions & Dilutions Worksheet
PPT Unit 4 The Mole PowerPoint Presentation, free download ID5601061
Answers Serial Dilutions Practice Worksheet 5 points each (2
Dilutions Worksheet Answers Thekidsworksheet
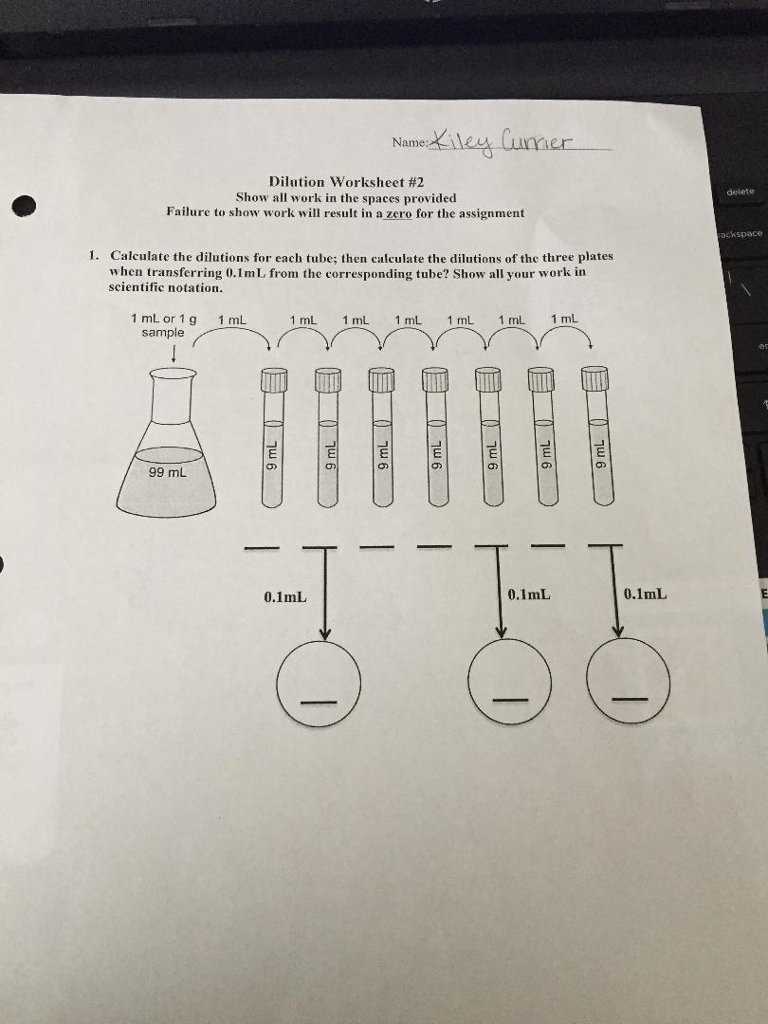
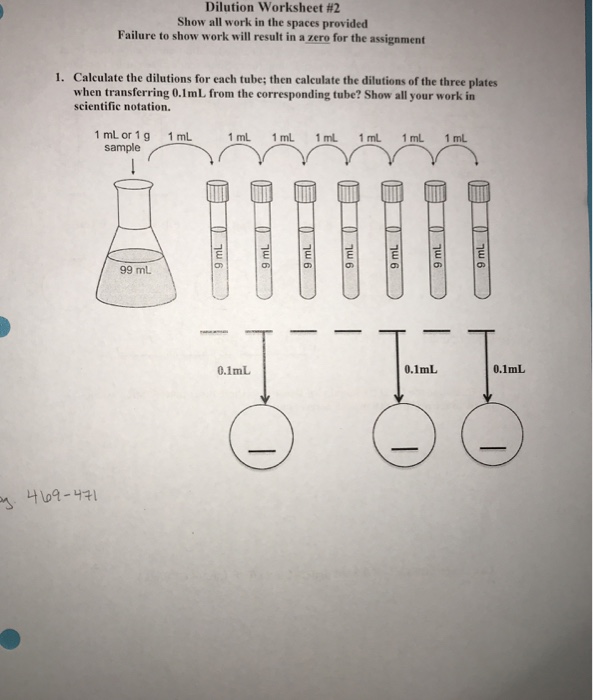
Solved Dilution Worksheet 2 Show all work in the spaces
7 Molarity Worksheet With Answers /
Calculating Percent By Mass Volume Chem Worksheet 15 2 Answers
Dilution worksheet keys Mrs. Morrison's "Flipped" Science Class
MCAT Question How to do Dilution Problems (M1V1 = M2V2) YouTube
Molarity Practice Worksheet Answer
If 455 Ml Of 6.0 M Hno3 Is.
Web For Example If You Have 5Ml Of A 2M Solution Which Is Diluted To A New Volume Of 10Ml The Molarity Will Be Reduced To 1M.
Web This Worksheet Features 5 Molarity Problems (M=Mol/L) With Conversions From Grams To Moles And Milliliters To Liters And 7 Dilutions Problems Using M1V1 = M2V2.
Web This Worksheet Features 5 Molarity Problems (M=Mol/L) With Conversions From Grams To.
Related Post: