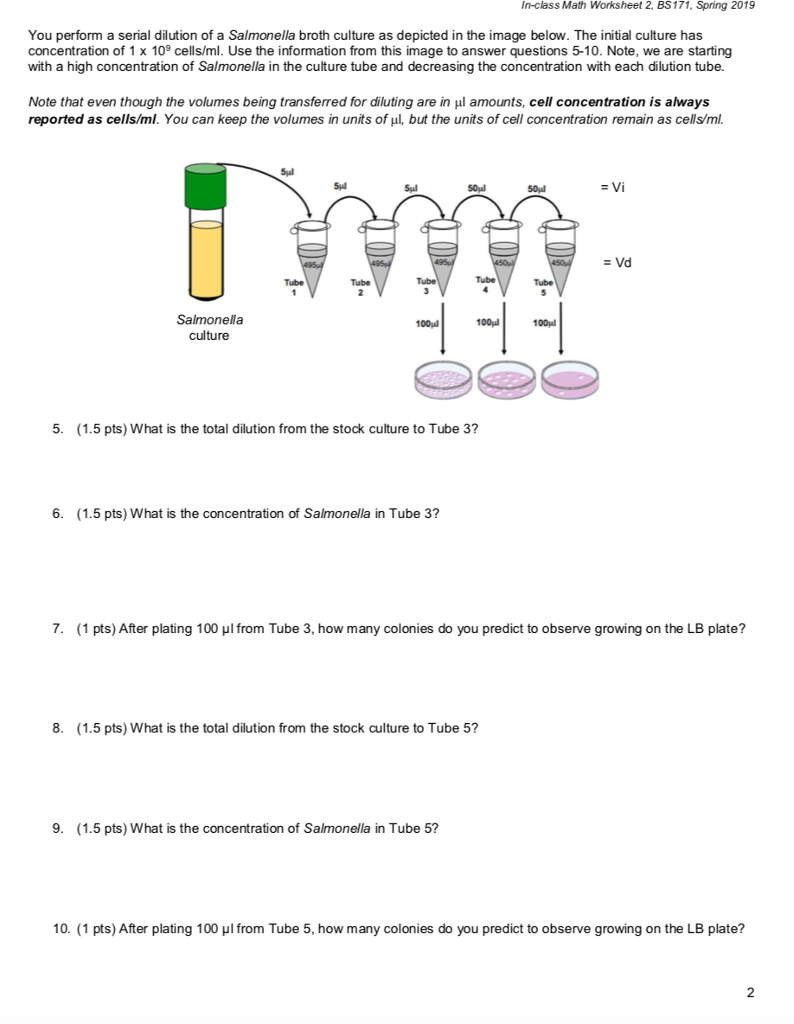
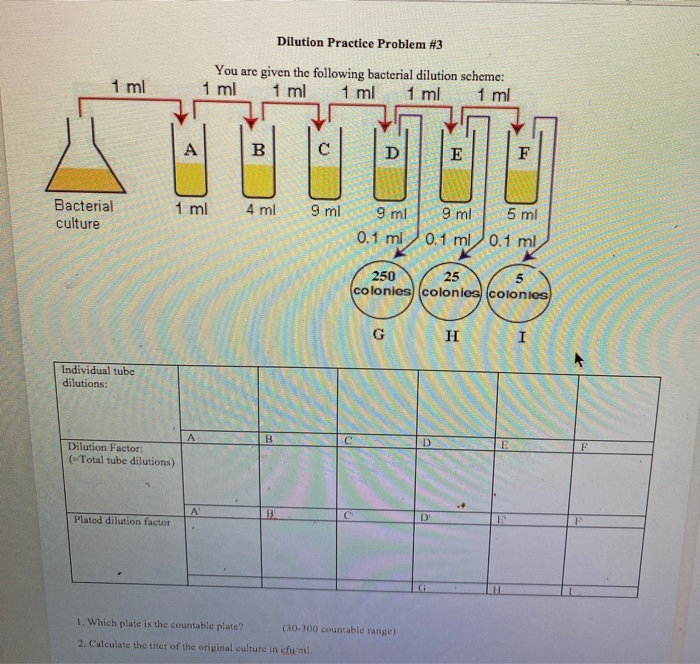
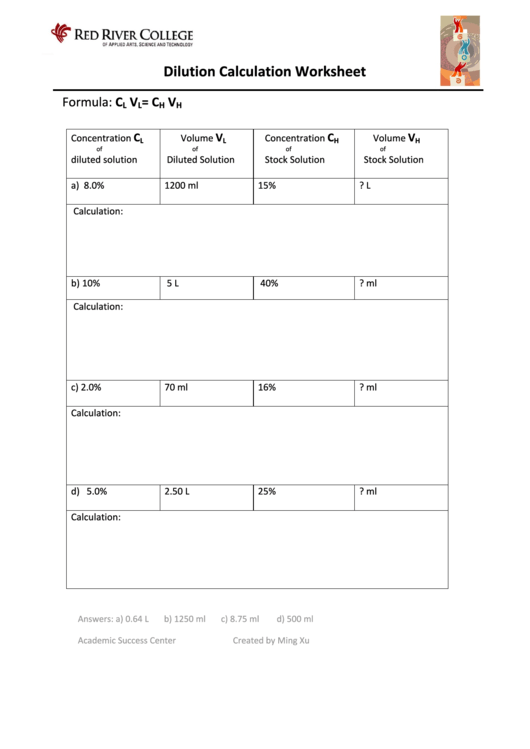
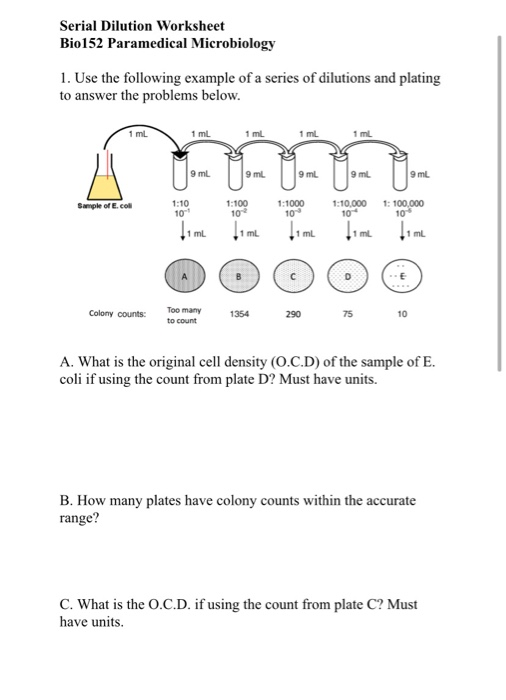
Dilution Problems Worksheet
Dilution Problems Worksheet - Boiling point elevation and freezing point depression. 2) if i add water to 100 ml of a 0.15 m naoh. Web problem #1:if you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. If 455 ml of 6.0 m hno3 is. Representing solutions using particulate models. Web the first three problems are questions regarding molarity and the others involve the dilutions formula above. Web dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Practice calculating molarity of a dilute solution with this 12 problem worksheet. This worksheet and quiz let you practice the following skills: Determine how many moles of solute and liters of solvent you have for the solution. Web dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 2) if i add water to 100 ml of a 0.15 m naoh. M1v1= m2v2 (1.6 mol/l) (175 ml) = (x) (1000 ml). Web dilution problems worksheet (m1v1 = m2v2) 1.. Web microbiology (biol 307) students shared 116 documents in this course. Own handwriting, with work shown when applic. The following problem sets test your ability to calculate dilution factors and concentration * s. Determine how many moles of solute and liters of solvent you have for the solution. Chemical nature of the reactants. Own handwriting, with work shown when applic. Web this process is known as dilution. Boiling point elevation and freezing point depression. A concentrated solution contains a relatively large amount of solute. Add the liters of solution and the liters of pure water together. Web dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Web dilution problems worksheet (m1v1 = m2v2) 1. Web to solve type one problems, first determine the individual dilution factor for each tube using the formula: Own handwriting, with work shown. Web a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Web to solve type one problems, first determine the individual dilution factor for each tube using the formula: How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Web. We can relate the concentrations and volumes before and after a dilution using the following equation: Web calculating final dilution in a given serial dilution problem skills practiced. Ed off of blackboar s must be wr. Boiling point elevation and freezing point depression. Web to solve type one problems, first determine the individual dilution factor for each tube using the. Web a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Web problem #1:if you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the. Determine the number of grams of nahco3 that are in one liter. Own handwriting, with work shown when applic. This worksheet and quiz let you practice the following skills: Web this process is known as dilution. M1v1= m2v2 (1.6 mol/l) (175 ml) = (x) (1000 ml). Boiling point elevation and freezing point depression. Representing solutions using particulate models. M₁v₁ = m₂v₂ where m₁ and v₁. Web microbiology (biol 307) students shared 116 documents in this course. Web this process is known as dilution. A concentrated solution contains a relatively large amount of solute. Determine the number of grams of nahco3 that are in one liter. This worksheet and quiz let you practice the following skills: Chemical nature of the reactants. Web this process is known as dilution. Web this worksheet defines dilution first, then students will complete 7 practice problems solving for both volume and concentration. We can relate the concentrations and volumes before and after a dilution using the following equation: Determine how many moles of solute and liters of solvent you have for the solution. Web dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Web this process is known as dilution. Web to solve type one problems, first determine the individual dilution factor for each tube using the formula: Practice calculating molarity of a dilute solution with this 12 problem worksheet. Web calculating final dilution in a given serial dilution problem skills practiced. A concentrated solution contains a relatively large amount of solute. Web microbiology (biol 307) students shared 116 documents in this course. Web problem #1:if you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Add the liters of solution and the liters of pure water together. Individual dilution factor = amount transferred. Web a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. 2) if i add water to 100 ml of a 0.15 m naoh. Boiling point elevation and freezing point depression. This worksheet and quiz let you practice the following skills: M1v1= m2v2 (1.6 mol/l) (175 ml) = (x) (1000 ml). The following problem sets test your ability to calculate dilution factors and concentration * s. Representing solutions using particulate models. We can relate the concentrations and volumes before and after a dilution using the following equation: Web to solve type one problems, first determine the individual dilution factor for each tube using the formula: Web dilution problems worksheet (m1v1 = m2v2) 1. Web this process is known as dilution. Representing solutions using particulate models. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Physical states of the reactants; The following problem sets test your ability to calculate dilution factors and concentration * s. Individual dilution factor = amount transferred. Ed off of blackboar s must be wr. M₁v₁ = m₂v₂ where m₁ and v₁. Own handwriting, with work shown when applic. Web a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Web problem #1:if you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Web dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? If 455 ml of 6.0 m hno3 is.Dilutions Worksheet Answers Heavy Wiring
Solved Dilutions Worksheet 1) If have 340 mL of a 0.5 M NaBr
Dilutions Worksheet Answer Key Thekidsworksheet
Solved Dilution Practice Problem 3 You are given the
Dilution Calculation Worksheet With Answers printable pdf download
Dilution Worksheet With Answers printable pdf download
Dilutions Worksheet
Dilutions Worksheet Answers Thekidsworksheet
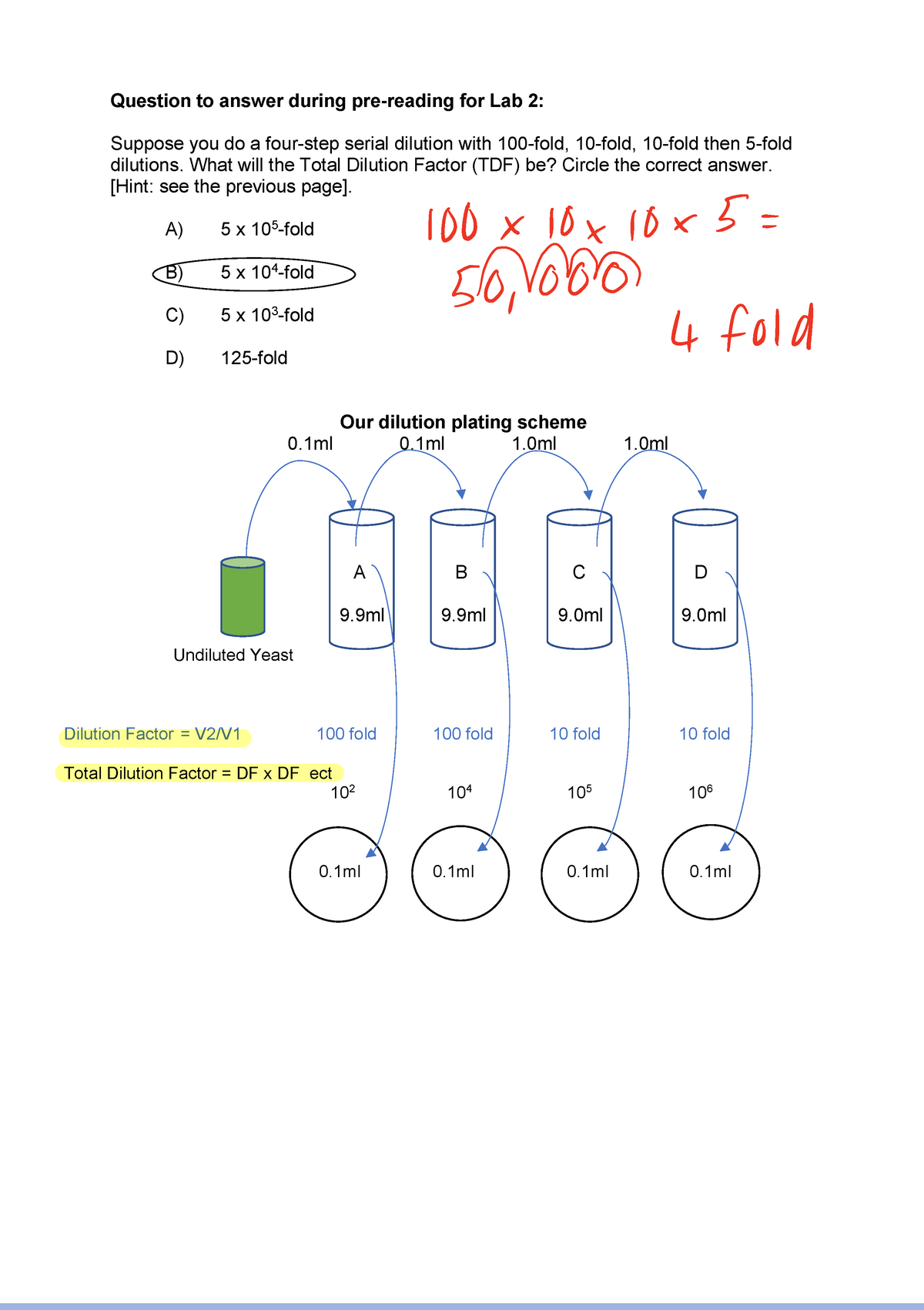
1. Serial Dilution Calculations Dilution Plating Questions Biology
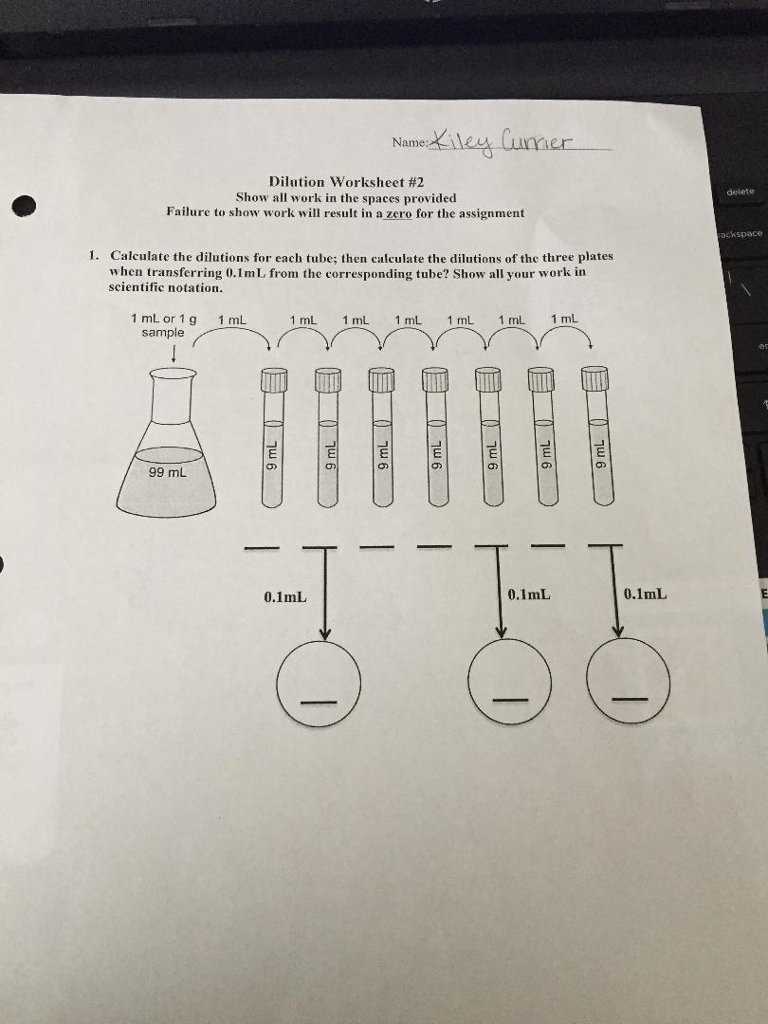
Solved Name Dilution Worksheet 2 Show all work in the
M1V1= M2V2 (1.6 Mol/L) (175 Ml) = (X) (1000 Ml).
Practice Calculating Molarity Of A Dilute Solution With This 12 Problem Worksheet.
Add The Liters Of Solution And The Liters Of Pure Water Together.
Web Calculating Final Dilution In A Given Serial Dilution Problem Skills Practiced.
Related Post: