Dilutions Worksheet Answer Key
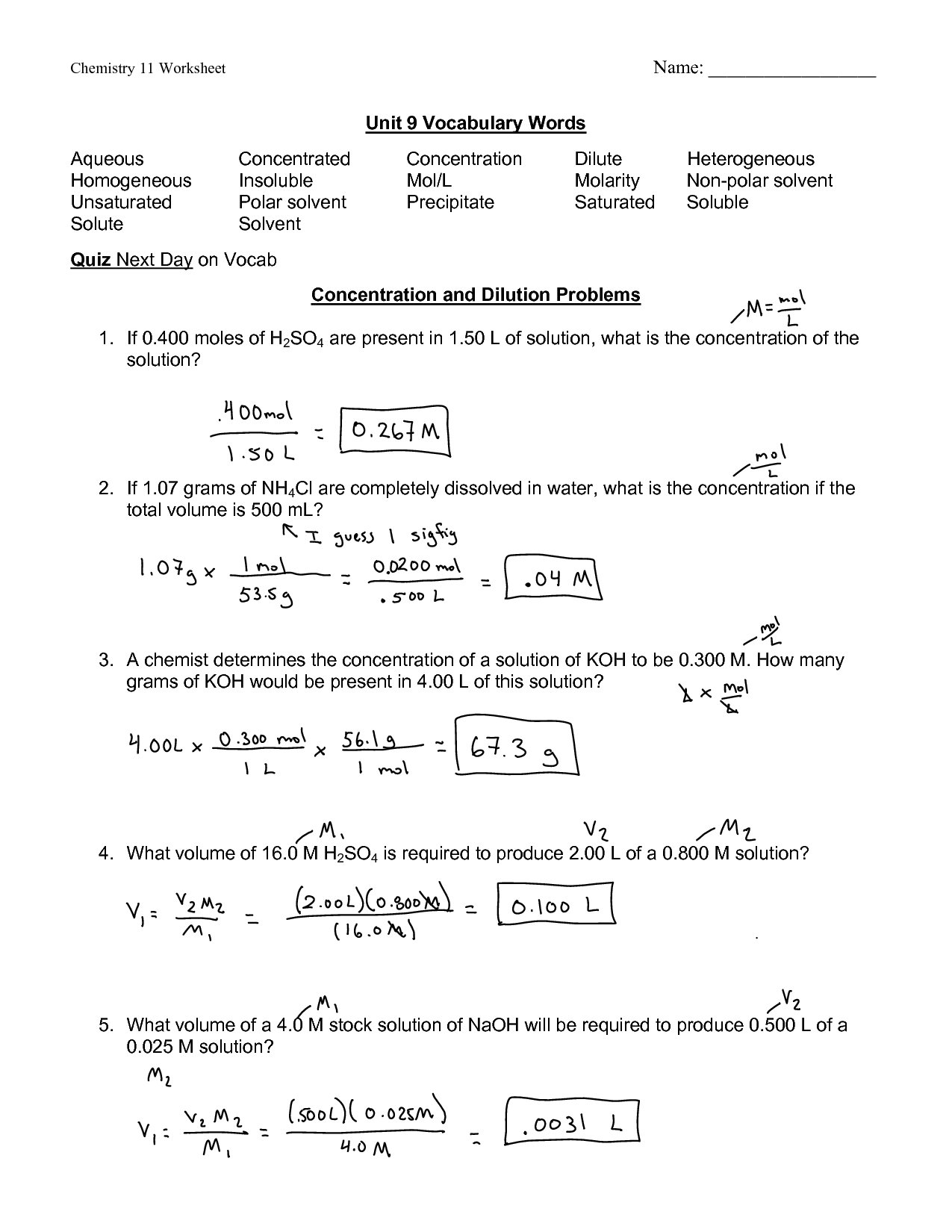
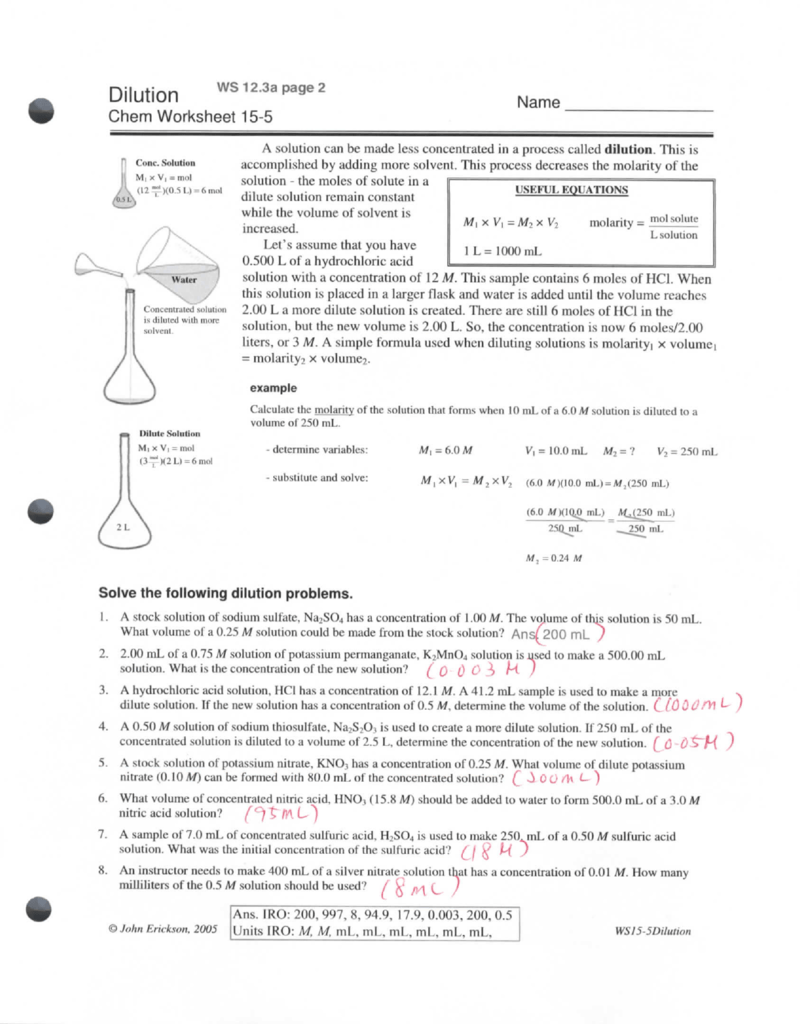
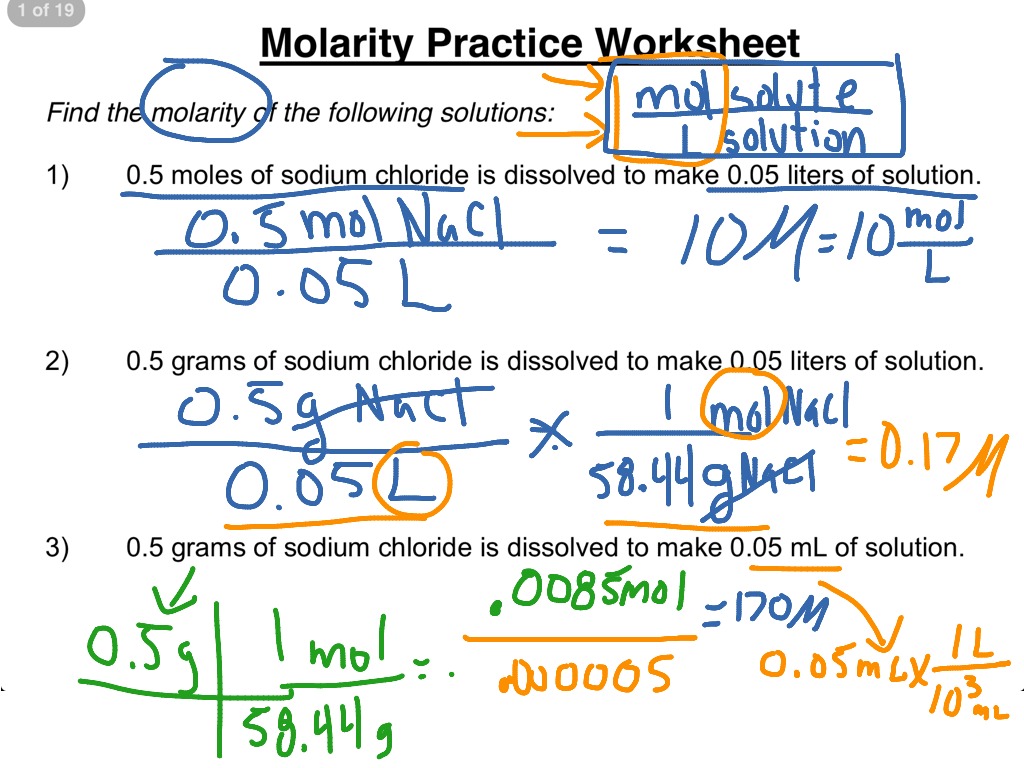
Dilutions Worksheet Answer Key - (2.500 mol/l) (100.0 ml) = (0.5500 mol/l) (x) x = 454.5 ml sometimes the problem might ask how much more. Own handwriting, with work shown when applic. Web do you need a digital resource for students to practice molarity by dilution problems? 1.) what is a solution? The concentration and the volumes change in a dilution. If i add 25 ml of water to 125 ml of a 0 m naoh solution, what will the molarity of the diluted solution be? A liquid solution is a homogeneous mixture of two or more substances and is produced when the solvent. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? Web this bundled product contains three worksheets with ten practice problems each involving molarity, dilutions, and molality. The number of moles always stays the same in a dilution. Understand how to quantify bacterial cells. The dilution is by a factor of 32 to go from 16 m to 0.5 m. Web think about your result. The concentration and the volumes change in a dilution. Ed off of blackboar s must be wr. It is a common practice to determine microbial counts for both liquid and solid. If i add 25 ml of water to 125 ml of a 0 m naoh solution, what will the molarity of the diluted solution be? Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions. These are the same directions that were on dilutions practice sheet. Learn how to solve a dilution problem. If you dilute 174 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of. Own handwriting, with work shown when applic. 0.25 l ( 250 ml) of the stock hno 3 needs to be diluted with. Web do you need a digital resource for students to practice molarity by dilution problems? Practice calculating molarity of a dilute solution with this 12 problem worksheet. Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions problems using m1v1=m2v2. For each of the following compounds, mark whether you. The number of moles always stays the same in a dilution. (2.500 mol/l) (100.0 ml) = (0.5500 mol/l) (x) x = 454.5 ml sometimes the problem might ask how much more. Learn how to solve a dilution problem. If you dilute 174 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of. It is. If i add 25 ml of water to 125 ml of a 0 m naoh solution, what will the molarity of the diluted solution be? These are the same directions that were on dilutions practice sheet. Own handwriting, with work shown when applic. If you dilute 174 ml of a 1.6 m solution of licl to 1.0 l, determine the. Own handwriting, with work shown when applic. Web placing the proper values into the dilution equation gives: Understand how to quantify bacterial cells. If you dilute 174 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of. Learn how to solve a dilution problem. If you dilute 174 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of. For each of the following compounds, mark whether you expect it to dissolve in water or not. Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions problems using. 1.) what is a solution? Understand how to quantify bacterial cells. The concentration and the volumes change in a dilution. ______key______ when a solution of glucose, c6h12o6, is diluted, the number of moles of the solute in the original solution is (greater than, less. Answer key included!check out all my resources for solutions:factors affecting solubility labsolutions vocab slidessolubility curve (graphs). Understand how to quantify bacterial cells. Learn how to solve a dilution problem. For each of the following compounds, mark whether you expect it to dissolve in water or not. If you dilute 174 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of. Web do you need a digital resource for students to. Understand how to quantify bacterial cells. Own handwriting, with work shown when applic. Learn how to solve a dilution problem. Web think about your result. If i add 25 ml of water to 125 ml of a 0 m naoh solution, what will the molarity of the diluted solution be? Web using a 4.0 m solution of mgso4 , determine how to make 300 ml of a 1.7 m dilution. Practice calculating molarity of a dilute solution with this 12 problem worksheet. Web answers m 1 v 1 = m 2 v 2 (1.71 m) (25.0 ml) = m 2 (65.0 ml) m 2 = 0.658 m m = mol/l = (25.0/40.0) / (0.325) = 1.92 mol/l g = (m) (l) (fw) = (0.400) ( (0.225) (119) = 10.7 g. The concentration and the volumes change in a dilution. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? M 1 v 1 = m 2 v 2 (0 m)(125 ml) = x (150 ml) x = 0 m. For each of the following compounds, mark whether you expect it to dissolve in water or not. Web up to 24% cash back dilutions worksheet i revised sunday, march 24, 2013 2 revision 1.0 name: Ed off of blackboar s must be wr. (2.500 mol/l) (100.0 ml) = (0.5500 mol/l) (x) x = 454.5 ml sometimes the problem might ask how much more. Calculate the molarity of 0.289 moles of fecl3 dissolved in 120 ml of solution? 0.25 l ( 250 ml) of the stock hno 3 needs to be diluted with water to a final volume of 8.00 l. If i add water to 100 ml of a 0 m naoh solution until the final volume is 150 ml, what will the molarity of the diluted solution be? Web this bundled product contains three worksheets with ten practice problems each involving molarity, dilutions, and molality. 1.) what is a solution? Learn how to solve a dilution problem. 0.25 l ( 250 ml) of the stock hno 3 needs to be diluted with water to a final volume of 8.00 l. For each of the following compounds, mark whether you expect it to dissolve in water or not. Web placing the proper values into the dilution equation gives: If you dilute 174 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of. This resource has ten problems with an included answer key. It is a common practice to determine microbial counts for both liquid and solid. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? 1.) what is a solution? These are the same directions that were on dilutions practice sheet. A liquid solution is a homogeneous mixture of two or more substances and is produced when the solvent. The concentration and the volumes change in a dilution. Own handwriting, with work shown when applic. The dilution is by a factor of 32 to go from 16 m to 0.5 m. Answer key included!check out all my resources for solutions:factors affecting solubility labsolutions vocab slidessolubility curve (graphs) notes & Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions problems using m1v1=m2v2.️Dilutions Worksheet 1 Answers Free Download Gambr.co
Making Dilutions Worksheet
Molarity And Dilution Phet Lab Answer Key » Judithcahen Answer Key For
7 Molarity Worksheet With Answers /
Dilution
molarity dilution worksheet
Molarity Worksheet Answer Key
Dilution worksheet keys Mrs. Morrison's "Flipped" Science Class
Concentration And Dilution Worksheet
Dilutions Worksheet Answer Key Chemistry
Web This Bundled Product Contains Three Worksheets With Ten Practice Problems Each Involving Molarity, Dilutions, And Molality.
______Key______ When A Solution Of Glucose, C6H12O6, Is Diluted, The Number Of Moles Of The Solute In The Original Solution Is (Greater Than, Less.
Understand How To Quantify Bacterial Cells.
Web Do You Need A Digital Resource For Students To Practice Molarity By Dilution Problems?
Related Post: