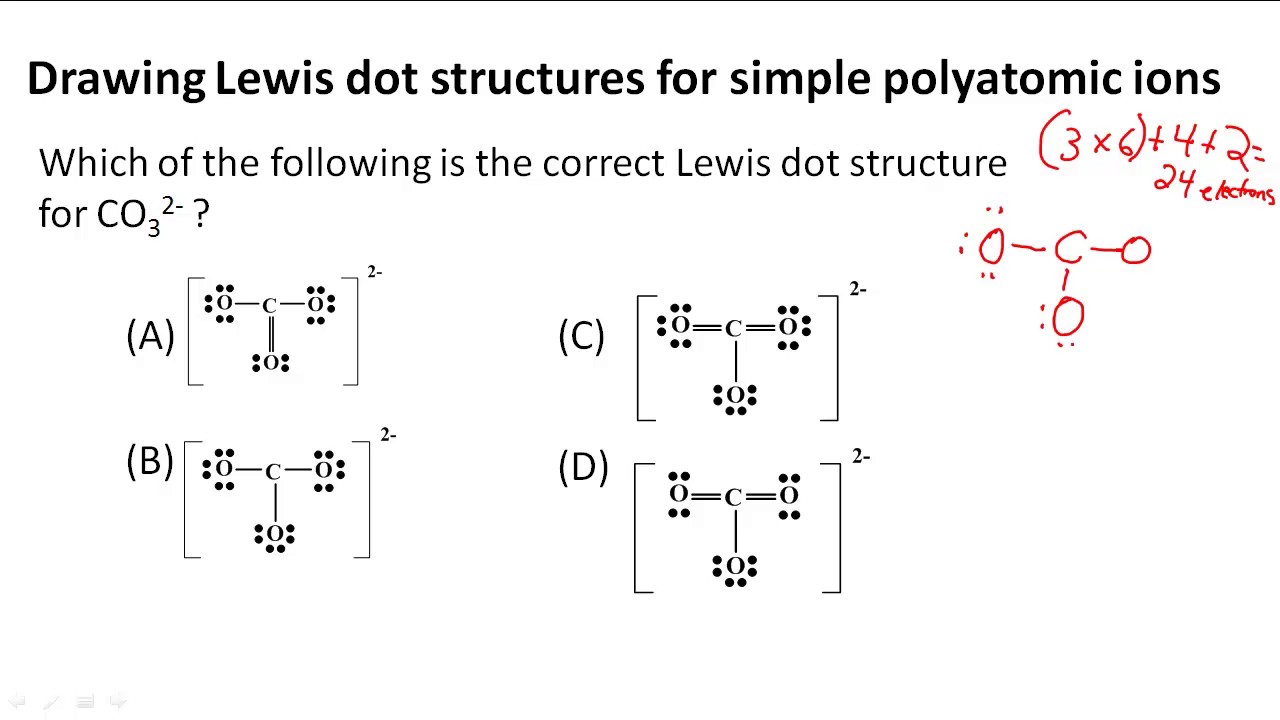
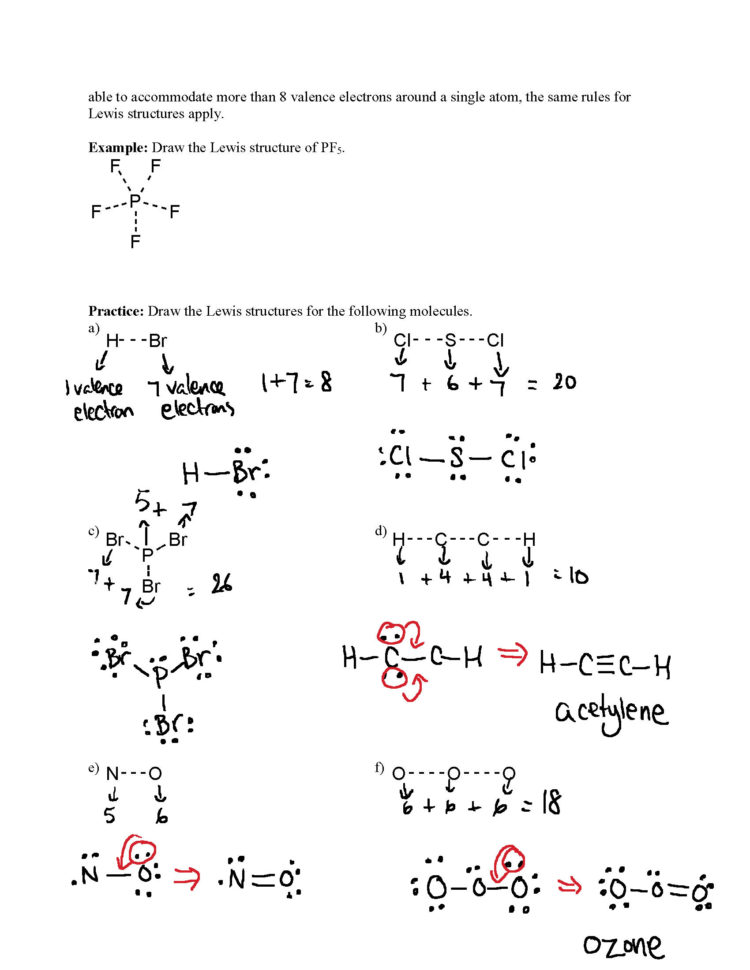
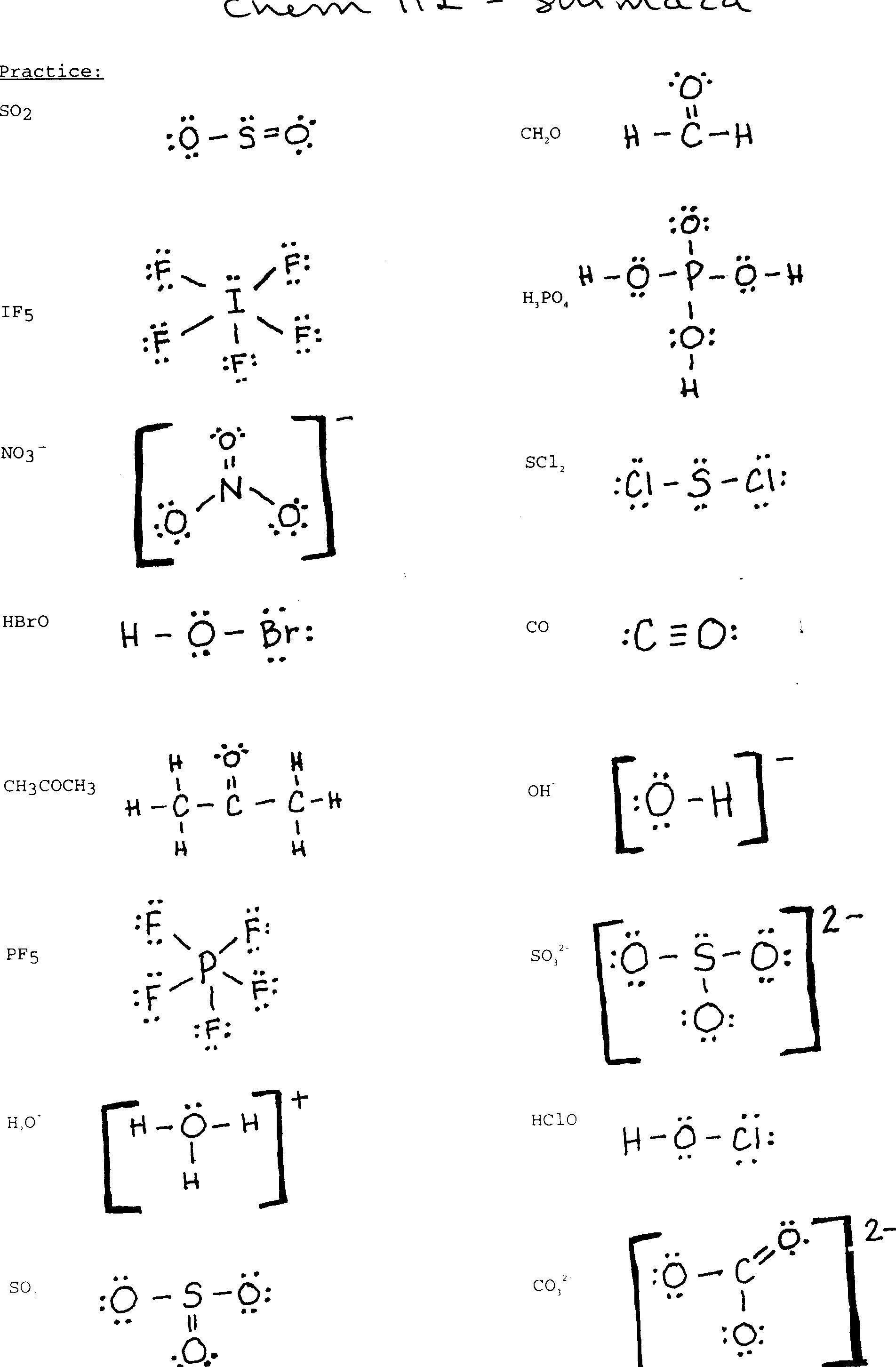
Drawing Lewis Structures Worksheet
Drawing Lewis Structures Worksheet - You should consult the lewis structure rules and. 1 4 5 6 7 normal # covalent bonds: Lewis diagram of xenon difluoride (xef₂) lewis diagrams. H c n o f/cl/br/i # valence electrons: Web the arrangement of atoms in several biologically important molecules is given here. Answers to these will be posted on the web late friday afternoon. If you get stuck, try asking another group for help. H o h a) how many groups (atoms and lone pairs) surround the central oxygen? Do not add any more atoms. Molecular and ionic compound structure and properties >. Determine the shape of the molecule. 1 4 5 6 7 normal # covalent bonds: They are to diagram the lewis electron dot structure for each, resulting in further insight on the configuration of different molecules. Incomplete octets 2 pcl group sf6 Web below are general, step by step rules to follow for drawing lewis structures of simple molecules. Web draw the lewis dot structure for each of the following polyatomic ions: Web instructions for drawing lewis #1 structures worksheet carefully and a: This is the first of two companion worksheets and in the molecular geometry we will start with the lewis dot structures of these molecules and determine their geometry's, polarity's and the types of molecular orbitals involved. H c n o f/cl/br/i # valence electrons: H o h a) how many groups (atoms and lone pairs) surround the central oxygen? Lewis diagram of formaldehyde (ch₂o) worked example: 4 b) what is the geometry of this molecule (look at atoms and lone pairs)? Web draw lewis structures for the following covalent compounds: If you get stuck, try asking another group for help. This is the first of two companion worksheets and in the molecular geometry we will start with the lewis dot structures of these molecules and determine their geometry's, polarity's and the types of molecular orbitals involved in the bonds. Ch3nh2 solutions to lewis structures homework 8. Determine the lewis structures. Complete the lewis structures of these molecules by adding multiple bonds and lone pairs. Draw the lewis diagram for each molecule. This is the first of two companion worksheets and in the molecular geometry we will start with the lewis dot structures of these molecules and determine their geometry's, polarity's and the types of molecular orbitals involved in the bonds.. H c n o f/cl/br/i # valence electrons: Ch 2 o (the carbon atom is the central atom.) one application of ch 2 o, also called formaldehyde, is the preservation of biological specimens. 1 4 5 6 7 normal # covalent bonds: Web the arrangement of atoms in several biologically important molecules is given here. If you are not sure. Determine the lewis structures of the following molecules: They are to diagram the lewis electron dot structure for each, resulting in further insight on the configuration of different molecules. Web draw the lewis structure for the following molecules. Do not add any more atoms. If more than one lewis structure can be drawn, use formal charges to decide on the. Simple molecules draw lewis sif4 structures for each group ch4 3 cloh2o period ______ of the following molecules: Draw the electron dot structure. Web practice these lewis structure on your own draw lewis structures for the following. There is a total of 3 + 3 x 7 = 24 electrons, and halogens on terminal positions are always going to have. Web practice these lewis structure on your own draw lewis structures for the following. If you are not sure if your structure is correct, do a formal charge check. If more than one lewis structure can be drawn, use formal charges to decide on the most preferred lewis structure. Web the following worksheet should be downloaded and filled out. Ia. There are the correct number of valence electrons. Web draw the lewis dot structure for each of the following polyatomic ions: Ch3nh2 solutions to lewis structures homework 8. Web below are general, step by step rules to follow for drawing lewis structures of simple molecules. Determine the approximate bond angles. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Give the name of the electronic arrangement and the name for the molecular geometry for each of the species in question #3. For each of the following, draw the lewis dot structure, give the electron arrangement (e.a.) and the molecular geometry (m.g.): There is a total of 3 + 3 x 7 = 24 electrons, and halogens on terminal positions are always going to have 3 lone pairs of electrons. Web instructions for drawing lewis #1 structures worksheet carefully and a: Web below are general, step by step rules to follow for drawing lewis structures of simple molecules. Also, helium is shown in group 8a, but it only has two valence electrons. Ia iva va via viia element: Draw the lewis diagram for each molecule. Determine valence electrons:determine the number of available valence electrons for the structure. Web another compound that has a triple bond is acetylene (c 2 h 2 ), whose lewis diagram is as follows: Web draw the lewis structure for the following molecules. They are to diagram the lewis electron dot structure for each, resulting in further insight on the configuration of different molecules. Web draw the best lewis dot structures for each of the following species. Answers to these will be posted on the web late friday afternoon. All structures follow the octet rule. Draw the lewis structure for water, h 2o. Web draw the lewis dot structure for each of the following polyatomic ions: Determine the lewis structures of the following molecules: Lewis diagram of xenon difluoride (xef₂) lewis diagrams. Determine the approximate bond angles. Determine valence electrons:determine the number of available valence electrons for the structure. An electron dot structure for an atom is simply the symbol for the element, surrounded by a number of dots equal to the number of valence electrons. This is simply the sum of the group numbers (from the periodic table) for all the atoms in the structure. Draw the lewis structure for water, h 2o. Web another compound that has a triple bond is acetylene (c 2 h 2 ), whose lewis diagram is as follows: Lewis diagram of formaldehyde (ch₂o) worked example: There are the correct number of valence electrons. This is the least electronegative atom (see back of periodic table for values) or the only one able to form more than one bond. Web draw the best lewis dot structures for each of the following species. For each of the following, draw the lewis dot structure, give the electron arrangement (e.a.) and the molecular geometry (m.g.): Web draw the lewis structure for the following molecules. Multiple bonds 2 group bcl3 d: Chemical formulatotal number of valence electronslewis dot structure. They are to diagram the lewis electron dot structure for each, resulting in further insight on the configuration of different molecules. You should try to answer the questions without referring to your textbook.Lewis Structures Worksheet With Answers
Lewis Dot Structure Practice Worksheet
Drawing Lewis Structures Worksheet
Lewis Dot Structure Worksheet
Drawing Lewis Structures Worksheet
Lewis Structure Worksheet 1 Answers
Drawing Lewis Structures Worksheet Lewis Structures Worksheet Free
Lewis Structure Worksheet With Answers Handmadefed
Lewis Structure Worksheet With Answers
Drawing Lewis Structures Worksheet
Incomplete Octets 2 Pcl Group Sf6
There Is A Total Of 3 + 3 X 7 = 24 Electrons, And Halogens On Terminal Positions Are Always Going To Have 3 Lone Pairs Of Electrons.
For Each Of The Following, Write The Formula, Draw The Lewis Dot Structure, And Draw The Model, And Determine The Type Of Bond.
Rules 1.) Identify The Central Atom Of The Molecule.
Related Post: