Electrochemical Cells Worksheet
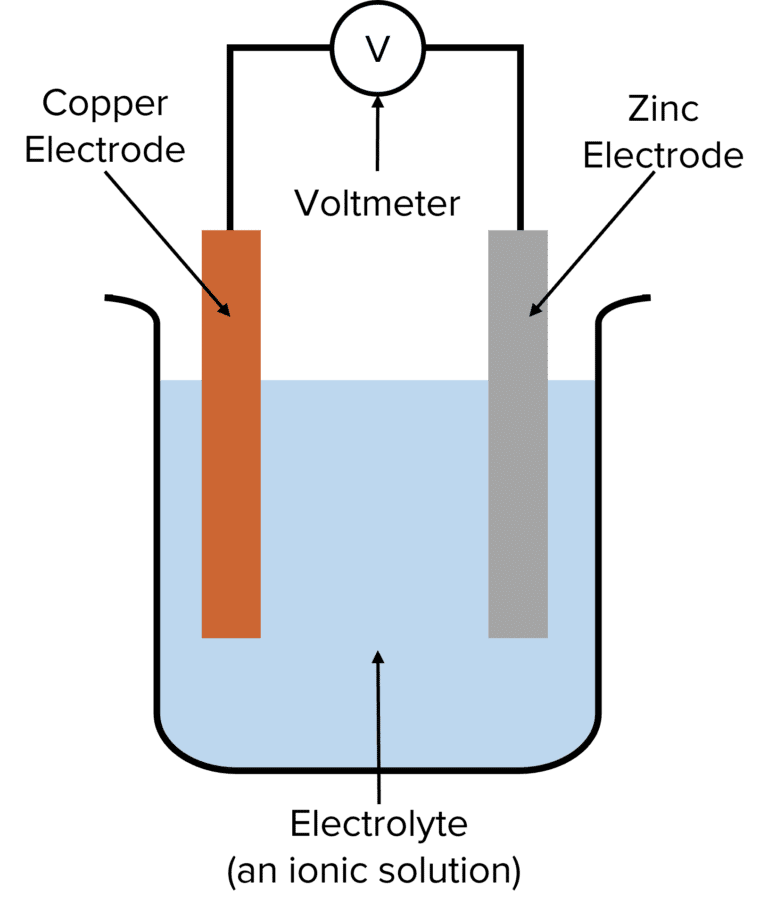
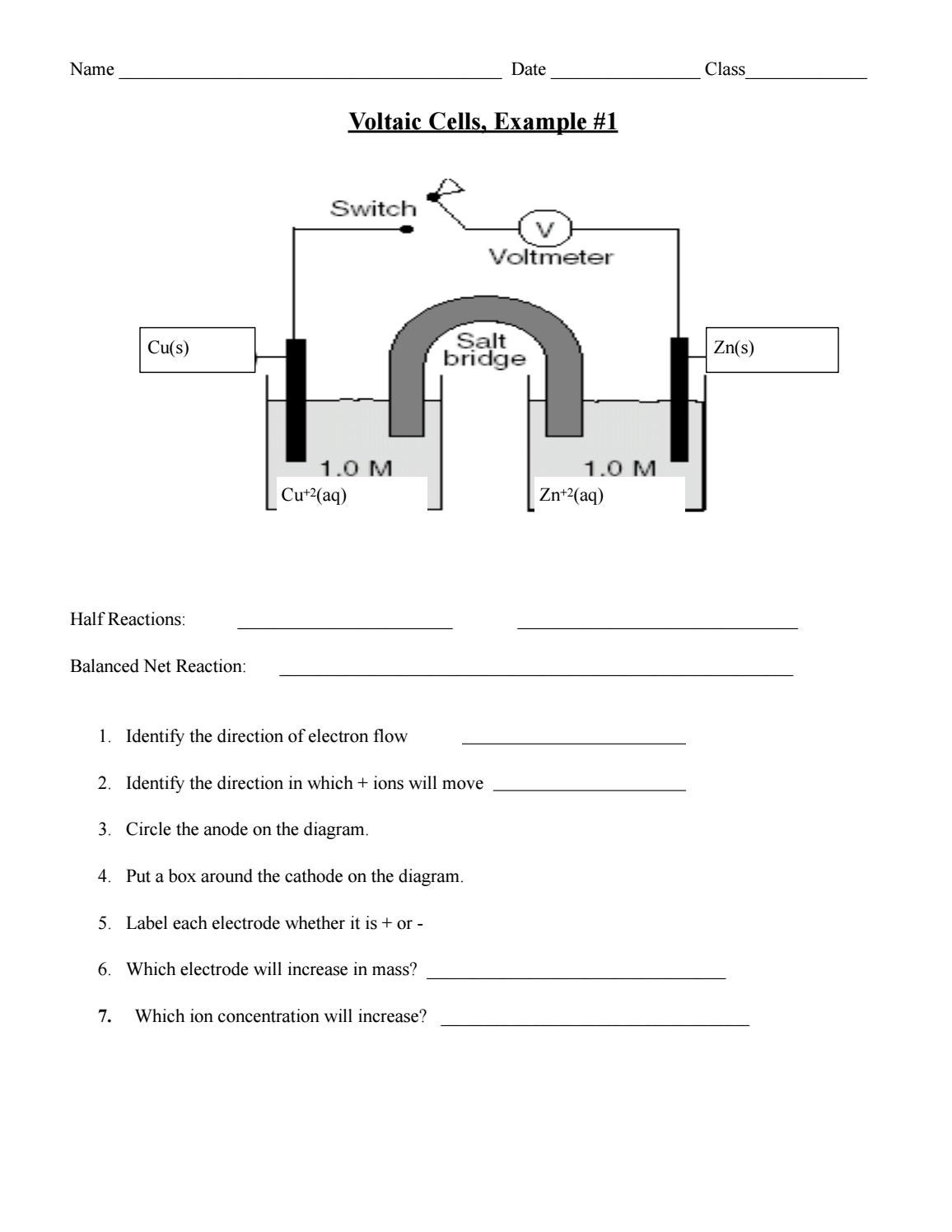
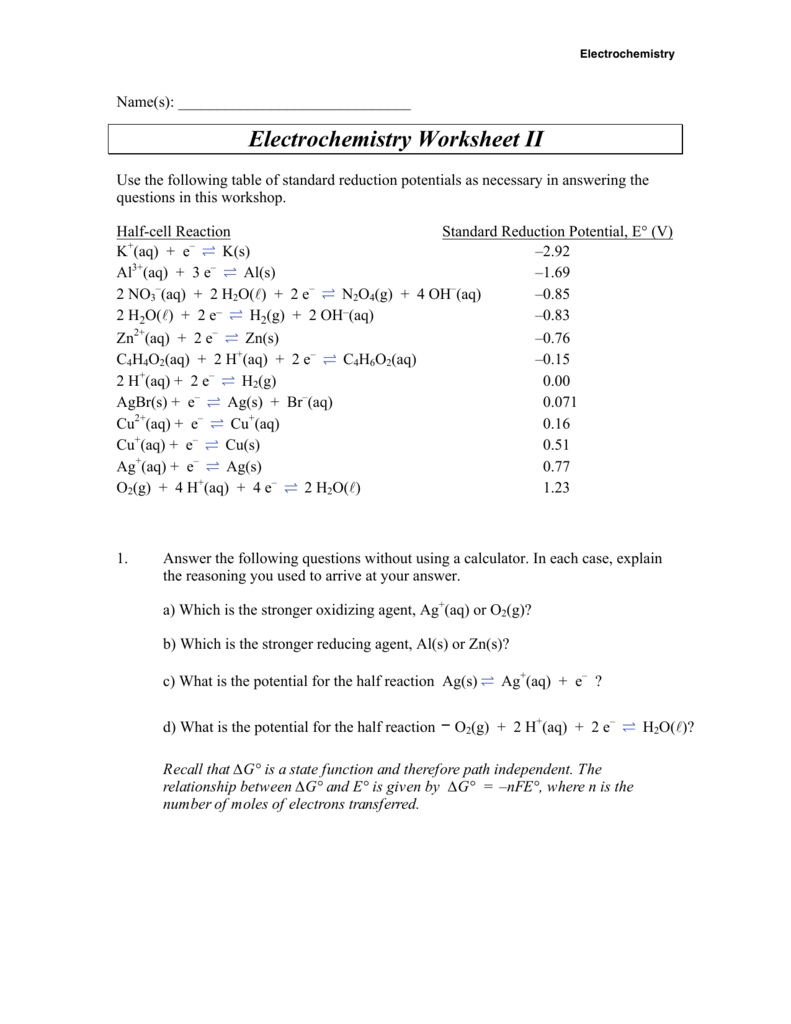
Electrochemical Cells Worksheet - Web revision on electrochemical cells for year 10 & 11. Which metal ion has the greater reduction potential? Web electrochemical cells worksheet 1. Calculate the standard cell potential produced by a galvanic cell consisting of a nickel electrode in contact with a solution of ni2+ ions and a. Web up to 24% cash back how an electrochemical cell works: Oxidation, or electron loss, occurs reduction, or the gaining of electrons, occurs current is generated voltage is. We examine the anatomy of batteries and light bulbs. Web electrochemical cells worksheet as you work through the steps in the lab procedures, record your experimental values and the results on this worksheet. Web these worksheets will explore all the different ways that use energy to power things or how we store it in the form of batteries. Web electrochemical cells worksheet as you work through the steps in the lab procedures, record your experimental values and theresults on this worksheet. Consider the voltaic cell that contains standard co2+/co and. Web calculate the standard cell potential produced by a galvanic cell consisting of a nickel electrode in contact with a solution of ni2+ ions and a silver electrode in contact with a. Web up to 24% cash back how an electrochemical cell works: 1.) consider this overall redox process: Web spontaneous. Web spontaneous voltaic electrochemical cells 17. Web electrochemical cells worksheet 1. What are all of the components of an electrochemical cell? A cathode, an anode, an electrolyte, and a wire. 1.) consider this overall redox process: When the concentration of znso 4 is 1.0 m and that of. These ions are released into the zinc sulfate solution. Web up to $3 cash back electrochemical cells worksheet. Oxidation, or electron loss, occurs reduction, or the gaining of electrons, occurs current is generated voltage is. At the anode, zn(s) atoms lose electrons to form zn2+ (aq) ions. Web spontaneous voltaic electrochemical cells 17. This resource bundle on electrochemical cells will test and challenge students. When the concentration of znso 4 is 1.0 m and that of. Some of the worksheets for this concept are electrochemical cells supplementary work experimental, work. These ions are released into the zinc sulfate solution. Which metal ion has the greater reduction potential? Cu 2+ (aq)+ba (s)→cu (s)+ba 2+ (aq) a.) split the reaction into half reactions and determine. Web up to $3 cash back electrochemical cells worksheet. Web electrochemical cells worksheet 1. Web electrochemistry worksheets class 12 chemistry. What are all of the components of an electrochemical cell? Of a daniell cell at 298 k is e 1. Oxidation, or electron loss, occurs reduction, or the gaining of electrons, occurs current is generated voltage is. Which metal ion has the greater reduction potential? Web calculate the standard cell potential produced by a galvanic cell consisting of a nickel. Web electrochemical cells worksheet as you work through the steps in the lab procedures, record your experimental values and the results on this worksheet. Oxidation, or electron loss, occurs reduction, or the gaining of electrons, occurs current is generated voltage is. Which statement about a voltaic cell is not correct? Web calculate the standard cell potential produced by a galvanic. Web electrochemical cells worksheet as you work through the steps in the lab procedures, record your experimental values and the results on this worksheet. A cathode, an anode, and a wire two compartments that conduct. Web electrochemical cells worksheet as you work through the steps in the lab procedures, record your experimental values and theresults on this worksheet. Calculate the. What are all of the components of an electrochemical cell? Of a daniell cell at 298 k is e 1. Some of the worksheets for this concept are electrochemical cells supplementary work experimental, work. Web electrochemical cells worksheet 1. Web spontaneous voltaic electrochemical cells 17. Web cell potential effective at a higher level learners correctly: Of a daniell cell at 298 k is e 1. This resource bundle on electrochemical cells will test and challenge students. Web electrochemical cells worksheet as you work through the steps in the lab procedures, record your experimental values and the results on this worksheet. Web these worksheets will explore. Which statement about a voltaic cell is not correct? Calculate the standard cell potential produced by a galvanic cell consisting of a nickel electrode in contact with a solution of ni2+ ions and a. Chemical species can have their oxidation number decreased at the cathode. A cathode, an anode, and a wire two compartments that conduct. Web up to $3 cash back electrochemical cells worksheet. At the anode, zn(s) atoms lose electrons to form zn2+ (aq) ions. A cathode, an anode, an electrolyte, and a wire. We examine the anatomy of batteries and light bulbs. Web revision on electrochemical cells for year 10 & 11. Web electrochemistry worksheets class 12 chemistry. Calculate the standard cell potential produced by a galvanic cell consisting of a nickel electrode in contact with a solution of ni 2+. Web spontaneous voltaic electrochemical cells 17. Web electrochemical cells worksheet as you work through the steps in the lab procedures, record your experimental values and the results on this worksheet. Web up to 24% cash back how an electrochemical cell works: Which metal ion has the greater reduction potential? If these two metals (and their solutions) were used to create a galvanic cell, which metal would be the anode? Web cell potential effective at a higher level learners correctly: Cu 2+ (aq)+ba (s)→cu (s)+ba 2+ (aq) a.) split the reaction into half reactions and determine. The anode of an electrochemical cell is the place where: Of a daniell cell at 298 k is e 1. Web these worksheets will explore all the different ways that use energy to power things or how we store it in the form of batteries. Which statement about a voltaic cell is not correct? Web up to 24% cash back how an electrochemical cell works: A cathode, an anode, and a wire two compartments that conduct. The anode of an electrochemical cell is the place where: Web revision on electrochemical cells for year 10 & 11. 1.) consider this overall redox process: Some of the worksheets for this concept are electrochemical cells supplementary work experimental, work. Which metal ion has the greater reduction potential? Chemical species can have their oxidation number decreased at the cathode. This resource bundle on electrochemical cells will test and challenge students. We examine the anatomy of batteries and light bulbs. At the anode, zn(s) atoms lose electrons to form zn2+ (aq) ions. What are all of the components of an electrochemical cell? These ions are released into the zinc sulfate solution. Web electrochemical cells worksheet 1.Electrochemical Cells Worksheets and Revision MME
Electrochemical Cell Worksheet by Olivia Hunter Issuu
Electrolytic cell worksheet 1 worksheet
the electrochemical cell worksheet
Electrochemistry Worksheet
Ejercicio de Electrolytic cell worksheet 3
electrochemical cells worksheet answers
Electrolytic cell worksheet
Redox Reactions Notes and Galvanic Cells Redox reactions, Chemistry
Electrolytic cell worksheet 1 Ficha interactiva
Web Up To $3 Cash Back Electrochemical Cells Worksheet.
If These Two Metals (And Their Solutions) Were Used To Create A Galvanic Cell, Which Metal Would Be The Anode?
Web Calculate The Standard Cell Potential Produced By A Galvanic Cell Consisting Of A Nickel Electrode In Contact With A Solution Of Ni2+ Ions And A Silver Electrode In Contact With A.
Of A Daniell Cell At 298 K Is E 1.
Related Post: