Electron Configuration Worksheet 2
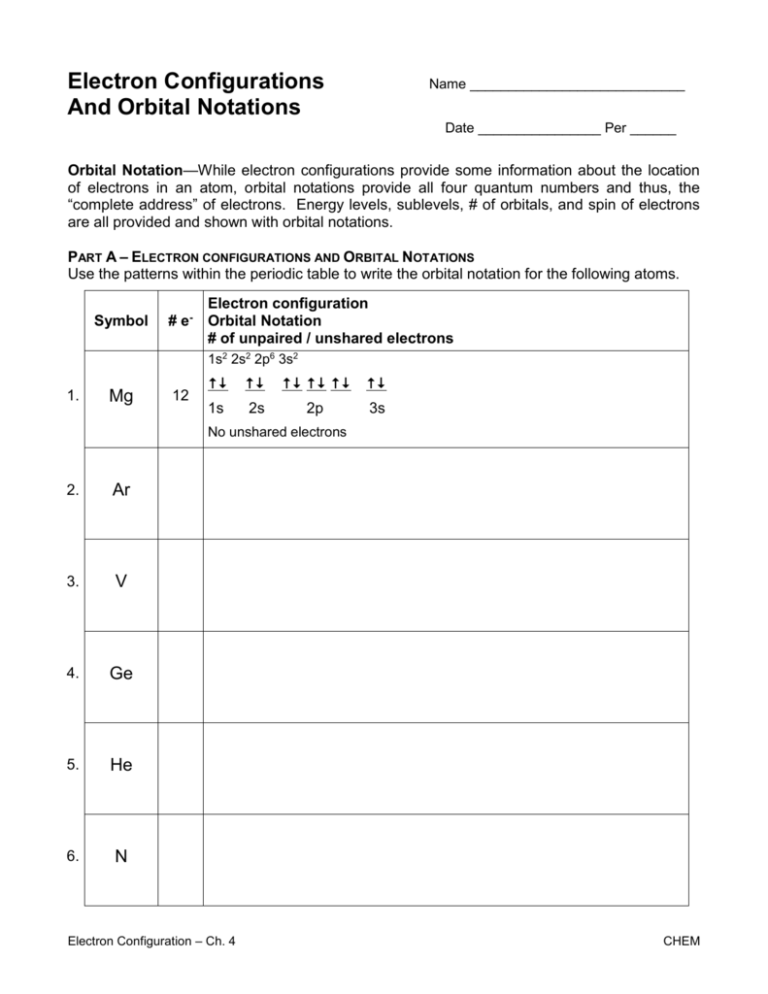
Electron Configuration Worksheet 2 - A number indicates the energy level (the number is called the principal quantum number.). 1) lithium 1s22s1 2) oxygen 1s22s22p4 3) calcium 1s22s22p63s23p64s2 4) titanium 1s22s22p63s23p64s23d2 5) rubidium 1s22s22p63s23p64s23d104p65s1 6) lead. It is labelled with a number (n) where n = 1, 2, 3 etc. An answer scheme is provided to allow for peer marking. Write the complete electron configuration for each isotope. Elements which have more than 20 protons are able to hold a. Some of the worksheets for this concept are 12 electron energy and light t, lewis electron dot structure answers, electron configuration work name vandenboutlabrake, electron configuration work, work 12, electron configuration practice work, atomic structure and electron configurations. A letter indicates the type of orbital; Web an electron configuration is a method of indicating the arrangement of electrons about a nucleus. Details of using the periodic table as a guide for determining electron configurations can be found on the ch301 website. A typical electron configuration consists of numbers, letters, and superscripts with the following format: Students will answer questions about energy levels, sublevels, and orbitals. This worksheet on electron configurations and the energy of electrons can be used as a quiz, a homework assignment, or review for a chapter test. Web determine if the following electron configurations are correct: Web this. An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. ______ 3) proton and neutron are the subatomic particles present in the nucleus. Suitable for grade 8 upward. Since there are 2 electrons in the box labeled 1s, we say the he electron configuration in orbital notation is 1s. 1s22s2 orbital notation + arrows: Web according to the rules of electronic configuration, two electrons can locate in the same orbital but with opposite spin directions. It is labelled with a number (n) where n = 1, 2, 3 etc. A shell is an energy level which electrons may occupy in an atom. The second and third levels each hold. Web brief instructions an electron configuration is a method of indicating the arrangement of electrons about a nucleus. The fourth level holds the remaining electrons. A number indicates the energy level (the number is called the principal quantum number.). 1s22s22p3 orbital notation + arrows: This worksheet on electron configurations and the energy of electrons can be used as a quiz,. A number indicates the energy level (the number is called the principal quantum number.). For the following atoms and ions: The electron configurations in this worksheet assume that lanthanum (la) is the first element in the 4f block and that actinium (ac) is the first element in the 5f block. Suitable for grade 8 upward. A typical electron configuration consists. Web the simple logic is that two arrows can go in each box, the first points up and the other points down. A shell is an energy level which electrons may occupy in an atom. The electron configurations in this worksheet assume that lanthanum (la) is the first element in the 4f block and that actinium (ac) is the first. The fourth level holds the remaining electrons. Elements which have more than 20 protons are able to hold a. Write the complete electron configuration for each isotope. A letter indicates the type of orbital; Write the ground state electron configuration of the following neutral elements in orbital notation, orbital notation with arrows and in short hand noble gas notation. For the following atoms and ions: Lithium (li) lithium li is element 3 with 3 electrons when it’s neutral. Web the simple logic is that two arrows can go in each box, the first points up and the other points down. It is labelled with a number (n) where n = 1, 2, 3 etc. ______ 4) electronegativity is the. Web according to the rules of electronic configuration, two electrons can locate in the same orbital but with opposite spin directions. Web ______ 1) electron is the kind of particle produced after covalent bond. 1s22s22p3 orbital notation + arrows: ______ 3) proton and neutron are the subatomic particles present in the nucleus. The easiest and most reliable technique for writing. Write the ground state electron configuration of the following neutral elements in orbital notation, orbital notation with arrows and in short hand noble gas notation. An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2. [he] 2s2 nitrogen orbital notation: Web this worksheet provides extra practice for writing electron configurations. ______ 4) electronegativity is the electron configuration of an atom or ion Since there are 2 electrons in the box labeled 1s, we say the he electron configuration in orbital notation is 1s 2. Co has 27 protons, 27 electrons, and 33 neutrons: Details of using the periodic table as a guide for determining electron configurations can be found on the ch301 website. The following electron configurations belong to which elements: An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7. Web this is a 2 page electron configuration worksheet that asks students to write electron configurations and orbital diagrams. Sodium 1s 2 2s 2 2p 6 3s 1 2)iron 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 3)bromine 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 I has 53 protons, 53 electrons, and 78 neutrons: It is labelled with a number (n) where n = 1, 2, 3 etc. Some of the worksheets for this concept are electron configuration practice work, electron configuration work, electron configuration practice work, chemistry 143 electron configurations caddell, atomic structure and electron configurations multiple, exam 4 collected essays, 13. Elements which have more than 20 protons are able to hold a. Web electron configuration elements (atoms) and ions. 1 1) 1s 2 2s 2 2p 6 3s 1 sodium 1 2) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p. Write the complete electron configuration for each isotope. A shell is an energy level which electrons may occupy in an atom. A number indicates the energy level (the number is called the principal quantum number.). A shell is an energy level which electrons may occupy in an atom. The electron configurations in this worksheet assume that lanthanum (la) is the first element in the 4f block and that actinium (ac) is the first element in the 5f block. An answer scheme is provided to allow for peer marking. 1) lithium 1s22s1 2) oxygen 1s22s22p4 3) calcium 1s22s22p63s23p64s2 4) titanium 1s22s22p63s23p64s23d2 5) rubidium 1s22s22p63s23p64s23d104p65s1 6) lead. A typical electron configuration consists of numbers, letters, and superscripts with the following format: The second and third levels each hold a maximum of eight electrons. It also includes a reference diagonal rule diagram, aufbau diagram, and periodic table.please examine the preview: The fourth level holds the remaining electrons. 1 1) 1s 2 2s 2 2p 6 3s 1 sodium 1 2) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p. Details of using the periodic table as a guide for determining electron configurations can be found on the ch301 website. Web electron configuration elements (atoms) and ions. Web according to the rules of electronic configuration, two electrons can locate in the same orbital but with opposite spin directions. Web this is a 2 page electron configuration worksheet that asks students to write electron configurations and orbital diagrams. Web determine if the following electron configurations are correct: A number indicates the energy level (the number is called the principal quantum number.). N is the principal quantum number.Electron Configuration Worksheets 2 Answer Key
Orbital Diagram Worksheet With Answers diagramwirings
Electron Configuration Practice Worksheet
Electron Configuration Worksheet
Electron Configuration Worksheet Answer Key Electronconfigurations
Electron configuration worksheet
Electron Configuration Orbital Diagrams Worksheet Answer Key Kayra Excel
√ Electron Configuration Practice Worksheet 2 Lir Stardust
Electron Configuration Worksheet Easy Hard Science
Electron Configuration Orbital Diagrams Worksheet Answer Key Kayra Excel
Web ______ 1) Electron Is The Kind Of Particle Produced After Covalent Bond.
______ 3) Proton And Neutron Are The Subatomic Particles Present In The Nucleus.
An Electron Configuration Diagram Is A Model That Depicts The Position Of Electrons As They Orbit The Nucleus Of An Atom.
Web The First Level Holds A Maximum Of Two Electrons.
Related Post: