Empirical And Molecular Formula Worksheet Answers
Empirical And Molecular Formula Worksheet Answers - Web empirical/molecular formula practice worksheet directions: Determining the absolute numbers of atoms that compose a. Write the empirical formula for the following compounds. Calculate the empirical formula of a. Write the empirical formula for the following compounds. 1) c 6 h 6 2) c 8. Students first calculate empirical formula using. Solution the molecular formula is c 6 h 12 o 6 because one molecule actually contains 6 c, 12 h, and 6 o. Web empirical and molecular formula worksheet. Composition, empirical formula and molecular formula worksheet sample of an unknown compound with mass of 0.847 has the following composition: The empirical formula for the compound having the formula h2c2o4 is [a] c2h2 [b] co2h [c] coh [d] c2o4h2 [e] coh2 2. Web empirical/molecular formula practice worksheet directions: Nicotine is an addictive compound found in tobacco leaves. Empirical formula = p2o5 empirical mass = 2(31.0) + 5(16.0) = 142g 283.89 ( 2 142 molecular mass = 283.89g. Composition, empirical formula. Web empirical/molecular formula practice worksheet directions: Write the empirical formula for the following compounds. Calculate the empirical formula of a. Write the empirical formula for the following compounds. Empirical formulae can be calculated from either % mass compostion data or mass composition data. Web empirical and molecular formula worksheet show work on a separate sheet of paper. Web the molecular formula of a compound is a representation of the number and type of elements present in one molecular unit of the compound. Elemental analysis of nicotine gives the following data: Web molecular formula worksheet answer key write the molecular formulas of the following. Empirical formula = p2o5 empirical mass = 2(31.0) + 5(16.0) = 142g 283.89 ( 2 142 molecular mass = 283.89g. 2 the mass of the empirical formula = (2 × 31) + (5 × 16) = 142 so the mass of the molecular formula is. N = 42.05g n ( 1 mol n ) = 3.001 mol n ( 14.01. N = 42.05g n ( 1 mol n ) = 3.001 mol n ( 14.01 g n) o. Write the empirical formula for the following compounds. 74.0 % c, 8.65 % h, 17.35 % n. Empirical formula = p2o5 empirical mass = 2(31.0) + 5(16.0) = 142g 283.89 ( 2 142 molecular mass = 283.89g. Solution the molecular formula is. Nicotine is an addictive compound found in tobacco leaves. Empirical formula = p2o5 empirical mass = 2(31.0) + 5(16.0) = 142g 283.89 ( 2 142 molecular mass = 283.89g. 2 the mass of the empirical formula = (2 × 31) + (5 × 16) = 142 so the mass of the molecular formula is. Write the empirical formula for the. Web up to $3 cash back answers to questions 1 the empirical formula is p2o5. Empirical formula = p2o5 empirical mass = 2(31.0) + 5(16.0) = 142g 283.89 ( 2 142 molecular mass = 283.89g. 1) a compound with an empirical formula of c 2 oh 4 and a. 2 the mass of the empirical formula = (2 × 31). Web molecular formula worksheet answer key write the molecular formulas of the following compounds: Web the molecular formula of a compound is a representation of the number and type of elements present in one molecular unit of the compound. Students first calculate empirical formula using. 74.0 % c, 8.65 % h, 17.35 % n. Write the empirical formula for the. Write the empirical formula for the following compounds. N = 42.05g n ( 1 mol n ) = 3.001 mol n ( 14.01 g n) o. Nicotine is an addictive compound found in tobacco leaves. What is the empirical formula. 74.0 % c, 8.65 % h, 17.35 % n. Web empirical and molecular formula worksheet. Empirical formulae can be calculated from either % mass compostion data or mass composition data. Web this activity worksheet examines both empirical formula and molecular formula through calculations and scaffolding steps. Web molecular formula worksheet answer key write the molecular formulas of the following compounds: Nicotine is an addictive compound found in tobacco leaves. Empirical formulae can be calculated from either % mass compostion data or mass composition data. Students first calculate empirical formula using. Write the empirical formula for the following compounds. 2 the mass of the empirical formula = (2 × 31) + (5 × 16) = 142 so the mass of the molecular formula is. Web what are the molecular and empirical formulas of glucose? Web up to $3 cash back answers to questions 1 the empirical formula is p2o5. Web solution to calculate percent composition, we divide the experimentally derived mass of each element by the overall mass of the compound, and then convert to. Web this activity worksheet examines both empirical formula and molecular formula through calculations and scaffolding steps. 1) a compound with an empirical formula of c 2 oh 4 and a. Web the molecular formula of a compound is a representation of the number and type of elements present in one molecular unit of the compound. 1) c 6 h 6 2) c 8. Web empirical and molecular formula worksheet. Determining the absolute numbers of atoms that compose a. Web empirical and molecular formula worksheet show work on a separate sheet of paper. N = 42.05g n ( 1 mol n ) = 3.001 mol n ( 14.01 g n) o. Empirical formula = ch empirical. Show work on a separate sheet of paper. 74.0 % c, 8.65 % h, 17.35 % n. Web empirical/molecular formula practice worksheet directions: Nicotine is an addictive compound found in tobacco leaves. Write the empirical formula for the following compounds. Web what are the molecular and empirical formulas of glucose? Write the empirical formula for the following compounds. Web empirical and molecular formula worksheet show work on a separate sheet of paper. Students first calculate empirical formula using. Web this activity worksheet examines both empirical formula and molecular formula through calculations and scaffolding steps. Solution the molecular formula is c 6 h 12 o 6 because one molecule actually contains 6 c, 12 h, and 6 o. 2 the mass of the empirical formula = (2 × 31) + (5 × 16) = 142 so the mass of the molecular formula is. Empirical formula = ch empirical. N = 42.05g n ( 1 mol n ) = 3.001 mol n ( 14.01 g n) o. Web empirical and molecular formula worksheet show work on a separate sheet of paper. Web molecular formula worksheet answer key write the molecular formulas of the following compounds: (if you were given % you would just change the % sign to a g for grams=easy!!) step 2: Nicotine is an addictive compound found in tobacco leaves. 1) a compound with an empirical formula of c 2 oh 4 and a. Determining the absolute numbers of atoms that compose a.Chemistry Empirical Formula Worksheets
11 Best Images of Molecular Formula Worksheet With Answers Molecular
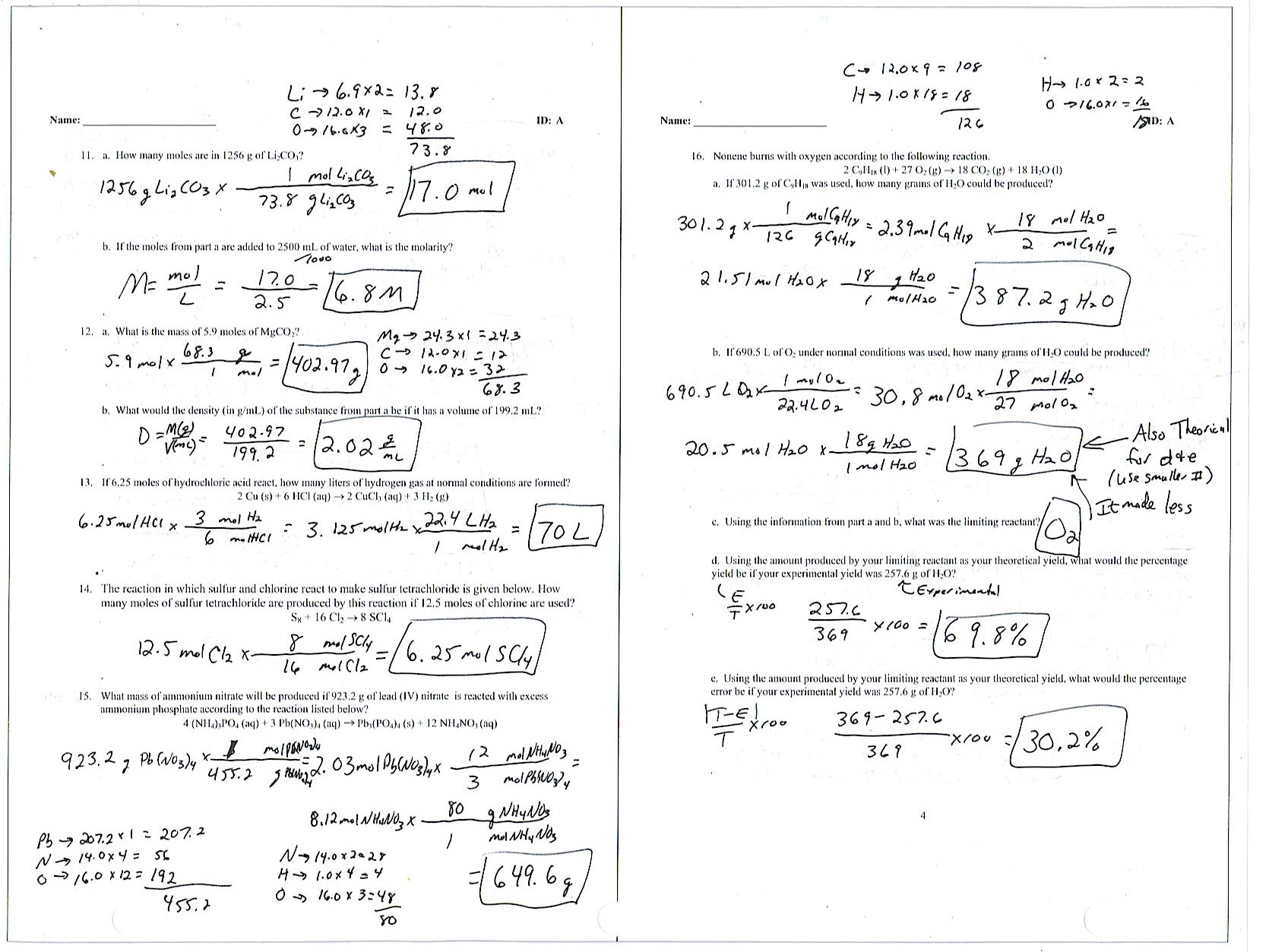
Chemistry Unit 4 Worksheet 2 Answers
Empirical Formula Worksheet Answers With Work Organicfer
Empirical Formula Worksheet 1 Answers Pdf Master of
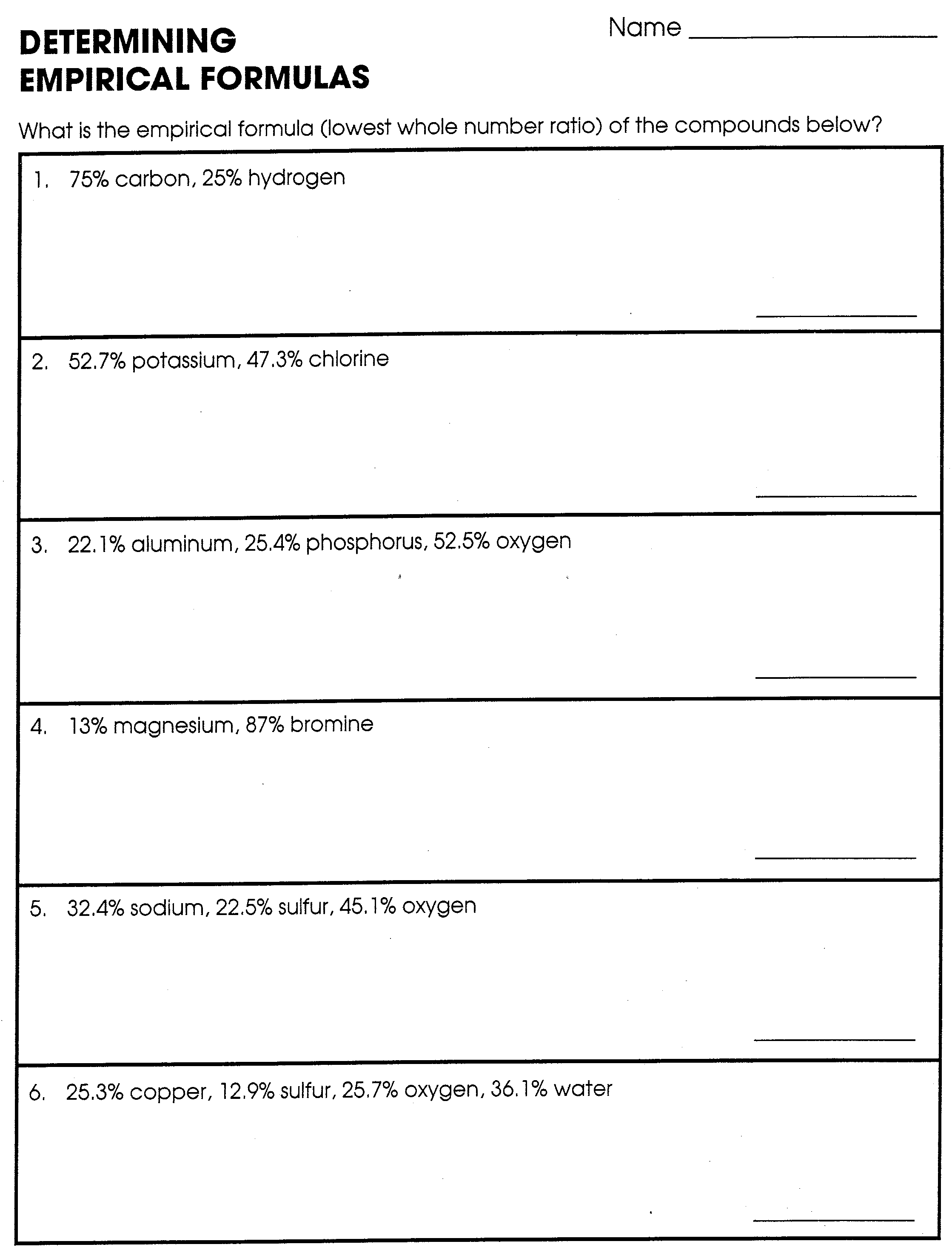
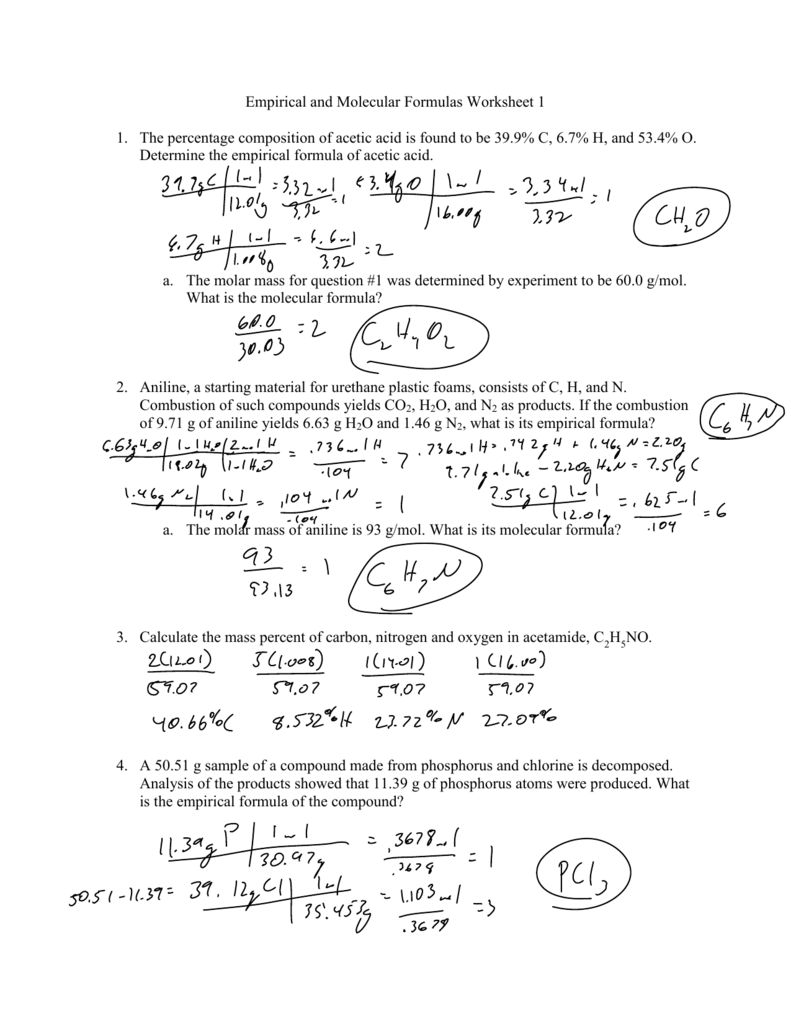
Empirical and Molecular Formulas Worksheet 1 1. The percentage
Chemistry Empirical Formula Worksheets
️Calculating Empirical Formula Worksheet Free Download Gambr.co
Empirical And Molecular Formula Worksheet 2 Herbalied
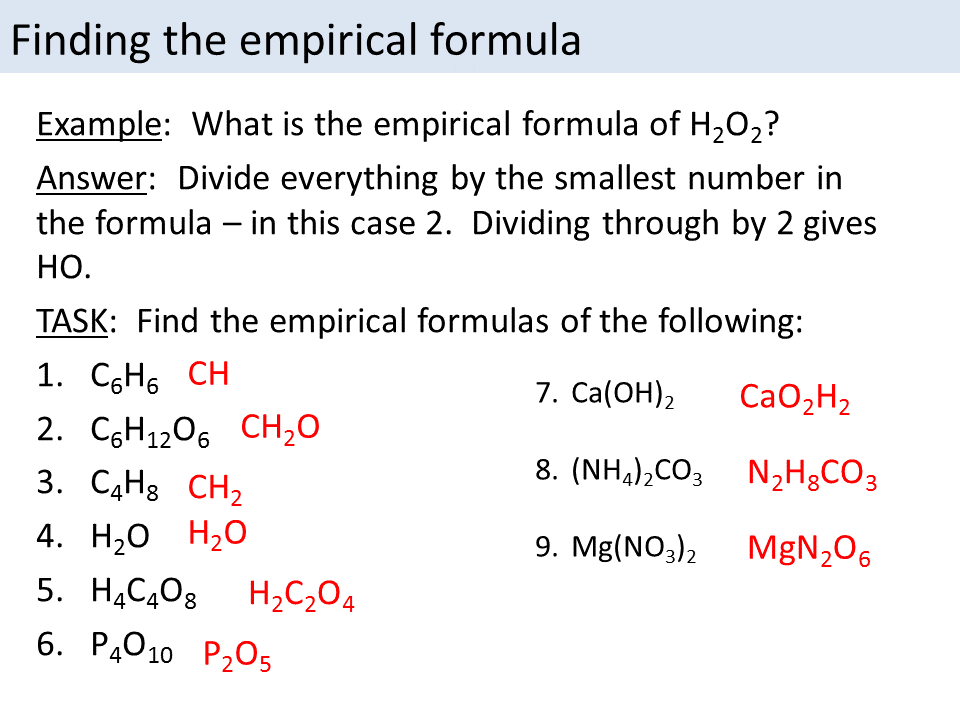
Empirical and Molecular Formula Molecules Chemical Compounds
Elemental Analysis Of Nicotine Gives The Following Data:
74.0 % C, 8.65 % H, 17.35 % N.
Composition, Empirical Formula And Molecular Formula Worksheet Sample Of An Unknown Compound With Mass Of 0.847 Has The Following Composition:
Web Empirical/Molecular Formula Practice Worksheet Directions:
Related Post: