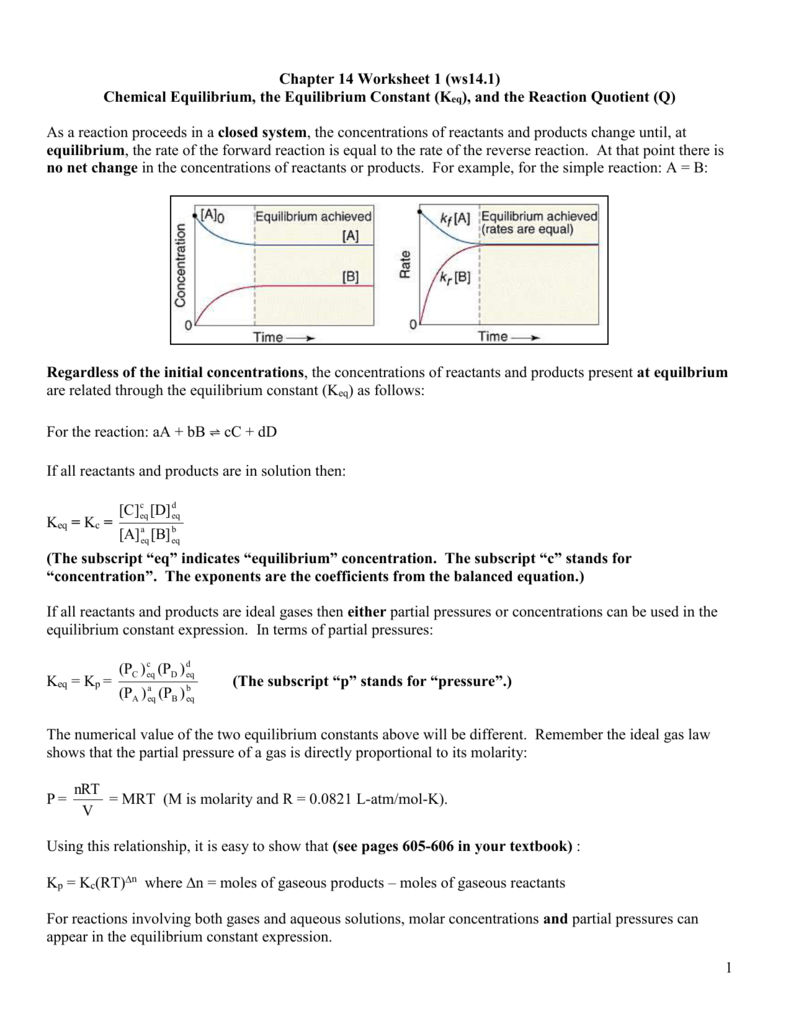
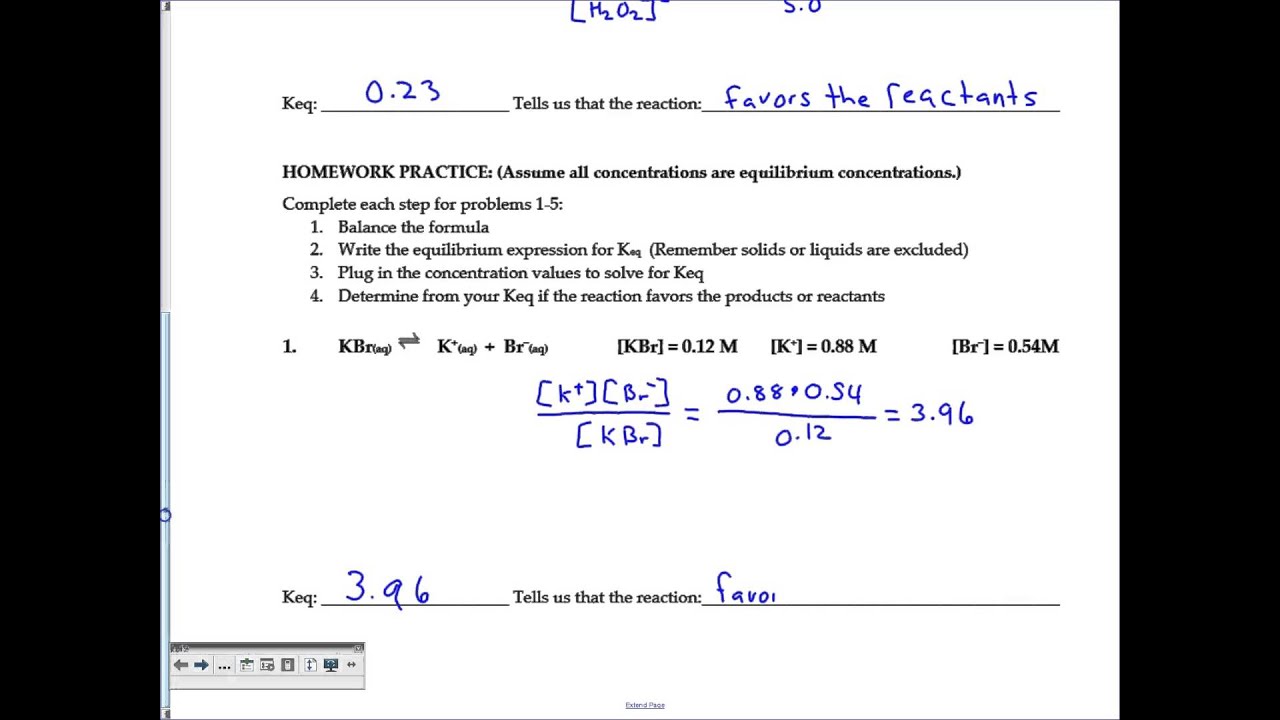
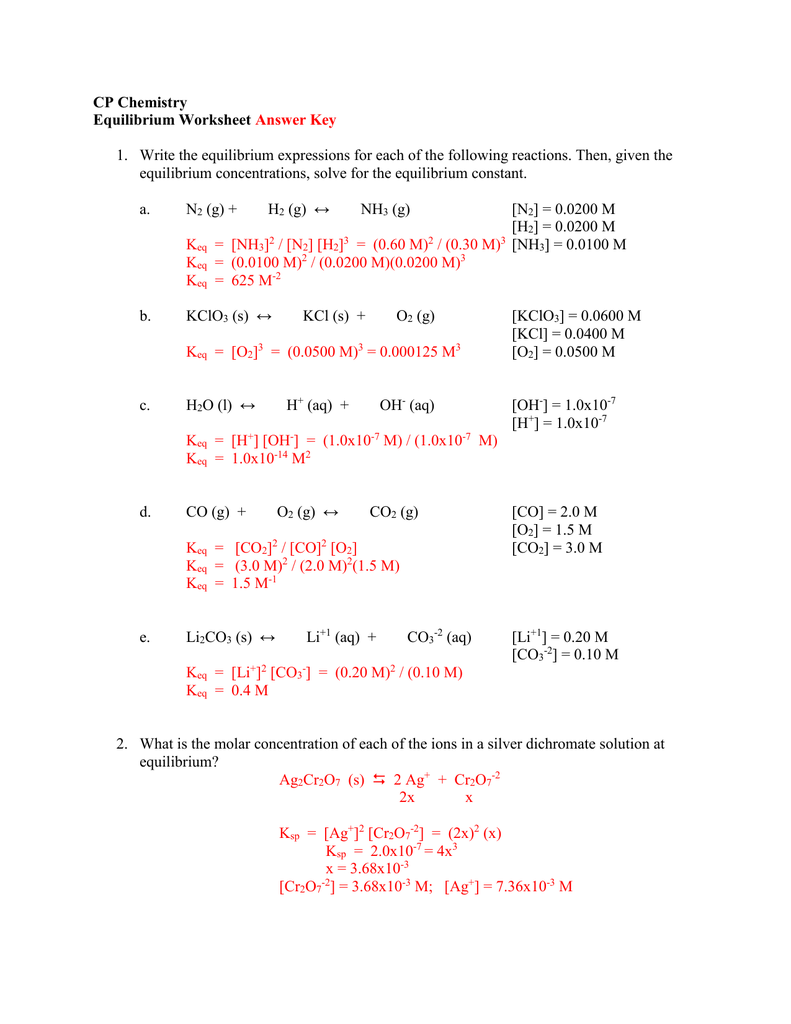
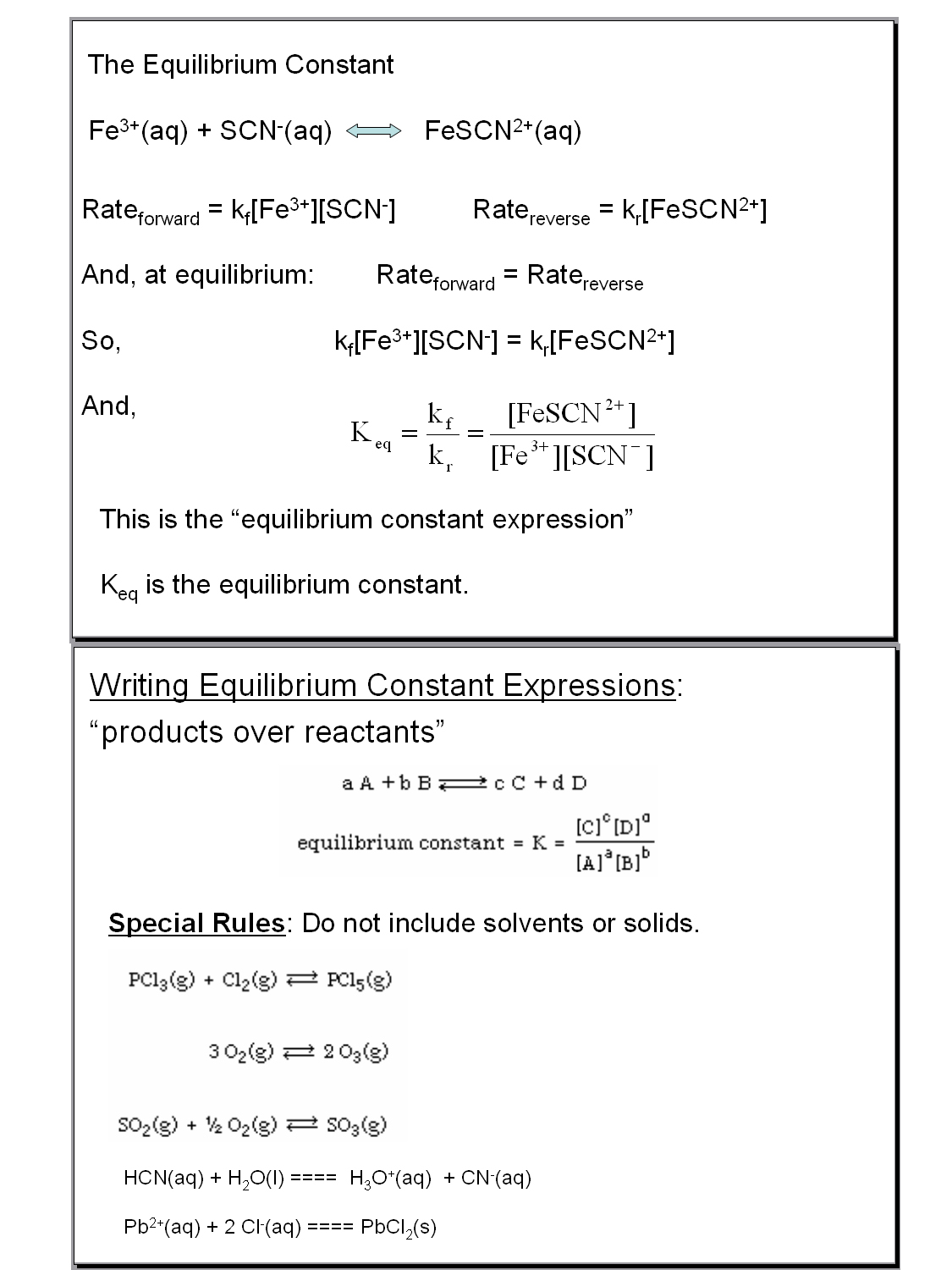
Equilibrium Constant Worksheet With Answers
Equilibrium Constant Worksheet With Answers - Web write the equilibrium expression for the reaction and calculate the value of the equilibrium constant for each experiment. Using this relationship, it is easy to show that Write the equilibrium constant expression for each of the following reactions: It relates the ratio of the concentrations of products to reactants once the reaction has reached chemical equilibrium. Calculate the equilibrium constant k for this reaction. The concentration of reactants and products are constant.(not equal) macroscopic properties are constant. The flask was found to contain 2.6 mol so 2. A) n 2 (g) + o 2 (g) ⇋ 2 no 2 (g) k = [ no 2 ] 2 [ n 2 ] [ o 2 ] 2. Web showing 8 worksheets for equilibrium constants. Write the equilibrium constant expression for each of the following reactions: The flask was found to contain 2.6 mol so 2. Worksheets are work 16, sample exercise writing equilibrium constant expressions, work 2 3 calculation. (a) i 2 is the catalyst; B) 2 c(s) + 3 h 2 (g) ⇋ c 2 h 6 (g) k = [ h 2 ] 3 [ c h 2 6] c) i 2 (aq). 5, [02] = 0.0500 m [co] = 2.0 m, = m, [c02] = 3.0m = 0.2 m, = m Calculations involving systems with very small or very large equilibrium constants can be dramatically simplified by making certain assumptions about the concentrations of products and reactants. Web write the equilibrium expression for the reaction and calculate the value of the equilibrium. K = 0.773 in the. Web at equilibrium, there is 0 mol of co, 0 mol of cl 2 and 1 mol of cocl 2 in a 2 l container. The ratio value is called the equilibrium constant (in terms of concentration), k c. A basic introduction to equilibrium constants should be given before hand including: It relates the ratio. Web calculations using the equilibrium constant name using the equilibrium constant expressions you determined on page 79, calculate the value of k when: The ratio value is called the equilibrium constant (in terms of concentration), k c. Web equilibrium constant worksheet 2 1. Worksheets are work 16, sample exercise writing equilibrium constant expressions, work 2 3 calculation. It relates. Calculate the equilibrium constant k for this reaction. (g) + 2 b (g) 3 c (g ans: How many moles of cl 2 must be added to reduce the concentration of co to 0 mol/l? It is always the same for a particular reaction under fixed conditions. Web keq = kp = (the subscript “p” stands for “pressure”.) the numerical. Remember the ideal gas law shows that the partial pressure of a gas is directly proportional to its molarity: A) n2 (g) + o2 (g) ⇋ 2 no2 (g) b) 2 c (s) + 3 h2 (g) ⇋ c2h6 (g) c) i2 (aq) ⇋ i2 (s) 2. Web write the equilibrium expression for the reaction and calculate the value of. Web showing 8 worksheets for equilibrium constants. Web keq = kp = (the subscript “p” stands for “pressure”.) the numerical value of the two equilibrium constants above will be different. Using this relationship, it is easy to show that [0 mol] at 350°c the equilibrium constant for the reaction caco3(s) ⇌. Web this discussion worksheet addresses equilibria and equilibrium constants. Worksheets are work 16, sample exercise writing equilibrium constant expressions, work 2 3 calculation. Web describe how to determine the magnitude of the equilibrium constant for a reaction when not all concentrations of the substances are known. Write the equilibrium constant expression for each of the following reactions: Forward rate is equal to the reverse rate. Worksheets are equilibrium constant. A basic introduction to equilibrium constants should be given before hand including: Which of the following is true about the reaction quotient? Web showing 8 worksheets for equilibrium constants. (color, mass, density, pressure, concentrations) 2 so 2 (g) + o 2 (g) ⇋ 2 so 3 (g) 4.0 mol of so 2 a n d 2.2 mol of o 2. Calculate the equilibrium constant k for this reaction. Web ch 102 discussion worksheet #7 summer 2023 1 answers to discussion worksheet 7 1. Web calculating equilibrium constant kc. The magnitude of k tells us the relative amounts (concentrations or pressures) of products to reactants once the reaction reaches. Using this relationship, it is easy to show that Using this relationship, it is easy to show that Web calculations using the equilibrium constant name using the equilibrium constant expressions you determined on page 79, calculate the value of k when: Web describe how to determine the magnitude of the equilibrium constant for a reaction when not all concentrations of the substances are known. Write the equilibrium constant expression for each of the following reactions: The law of mass action, reaction. [0 mol] at 350°c the equilibrium constant for the reaction caco3(s) ⇌. It relates the ratio of the concentrations of products to reactants once the reaction has reached chemical equilibrium. Web equilibrium constant worksheet 2 1. [nh3] = 0.0100 m, = 0.0200 m, = 0.0200 m kid xlò 2, 3. Web write the equilibrium expression for the reaction and calculate the value of the equilibrium constant for each experiment. (a) i 2 is the catalyst; K = 0.774 expt 2: A) n 2 (g) + o 2 (g) ⇋ 2 no 2 (g) k= [no2] 2 [n2][o] 2 b) 2 c(s) + 3 h 2 (g) ⇋ c 2 h 6 (g) k= [h2] 3 [c2h6] c) i 2 (aq) ⇋ i 2 (s) k= 1 Web calculating equilibrium constant kc. Web calculate the equilibrium constant for the reaction: Remember the ideal gas law shows that the partial pressure of a gas is directly proportional to its molarity: Calculations involving systems with very small or very large equilibrium constants can be dramatically simplified by making certain assumptions about the concentrations of products and reactants. Web calculate the equilibrium constant for the reaction: Web showing 8 worksheets for equilibrium constants. 12 (g) + br2 (a) assume that equilibrium is established at the above temperature by adding o i (g) to the reaction flask. K = 0.774 expt 2: 5, [02] = 0.0500 m [co] = 2.0 m, = m, [c02] = 3.0m = 0.2 m, = m 12 (g) + br2 (a) assume that equilibrium is established at the above temperature by adding o i (g) to the reaction flask. The flask was found to contain 2.6 mol so 2. Web write the equilibrium expression for the reaction and calculate the value of the equilibrium constant for each experiment. Forward rate is equal to the reverse rate. (a) i 2 is the catalyst; A) n 2 (g) + o 2 (g) ⇋ 2 no 2 (g) k = [ no 2 ] 2 [ n 2 ] [ o 2 ] 2. K = 0.773 in the. Web calculate the equilibrium constant for the reaction: Web calculate the equilibrium constant for the reaction: K = 0.774 expt 3: A) n2 (g) + o2 (g) ⇋ 2 no2 (g) b) 2 c (s) + 3 h2 (g) ⇋ c2h6 (g) c) i2 (aq) ⇋ i2 (s) 2. Web keq = kp = (the subscript “p” stands for “pressure”.) the numerical value of the two equilibrium constants above will be different. [0 mol] at 350°c the equilibrium constant for the reaction caco3(s) ⇌. Using this relationship, it is easy to show thatPressure Equilibrium Constant Expression sharedoc
Calculations Using The Equilibrium Constant Worksheet Answers ZHISHU WEB
Equilibrium Constant Worksheet Worksheets For Kindergarten
equilibriumkey (1)
Calculating Equilibrium Constants Chem Worksheet 18 3 Answers
CP Chemistry
Equilibrium Constant Worksheet. Worksheets. Tutsstar Thousands of
CHEMISTRY 12 WORKSHEET (Keq Level 3 Questions)
equilibrium constant worksheet with answers
Equilibrium 7.57.6 practice answers
B) 2 C(S) + 3 H 2 (G) ⇋ C 2 H 6 (G) K = [ H 2 ] 3 [ C H 2 6] C) I 2 (Aq) ⇋ I 2 (S) K = 1 [ I 2 ( Aq )]
A Basic Introduction To Equilibrium Constants Should Be Given Before Hand Including:
Web Equilibrium Constant Worksheet 2 1.
Web The Equilibrium Constant, K, Is Used To Determine The Relative Concentrations Of Products And Reactants At Equilibrium.
Related Post: