Gas Law Stoichiometry Worksheet
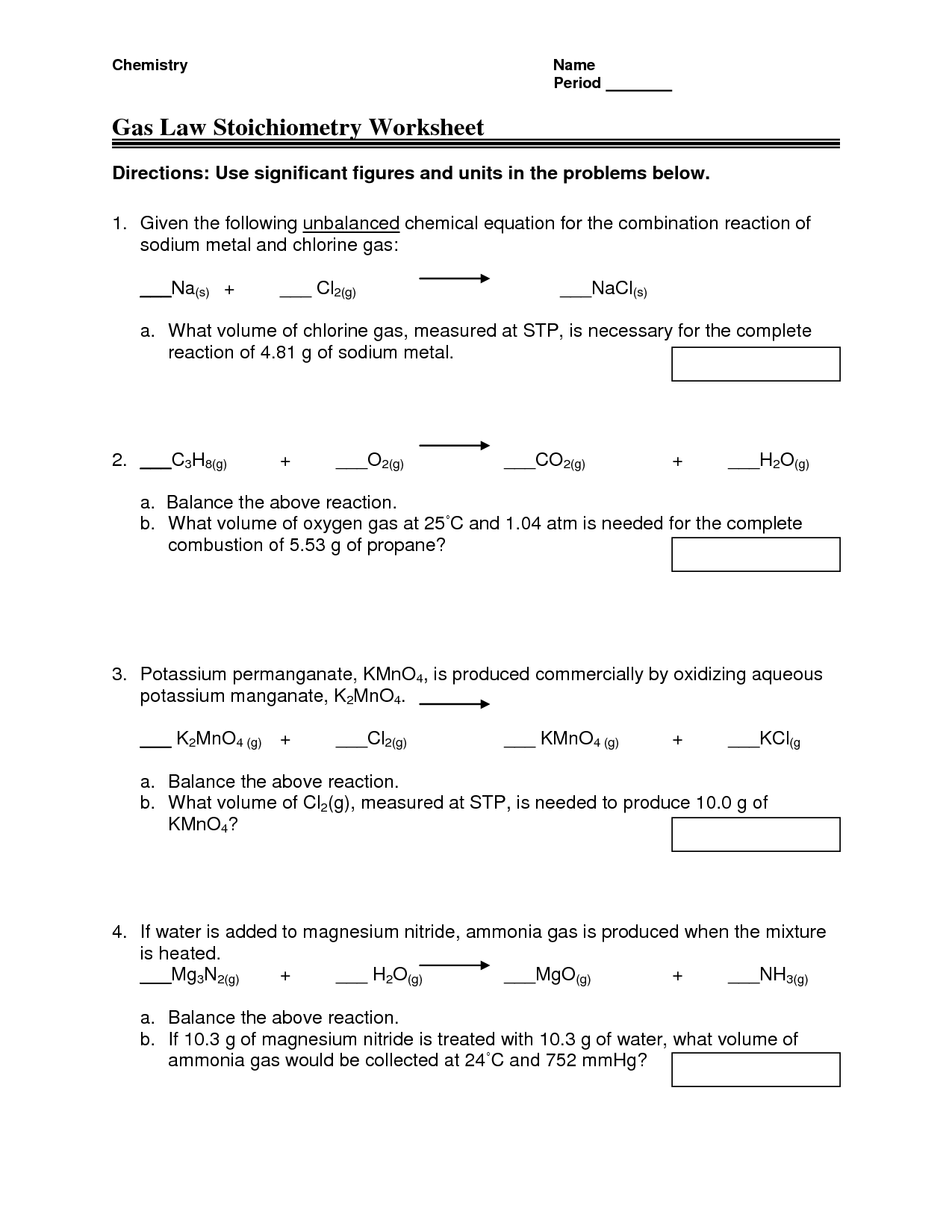
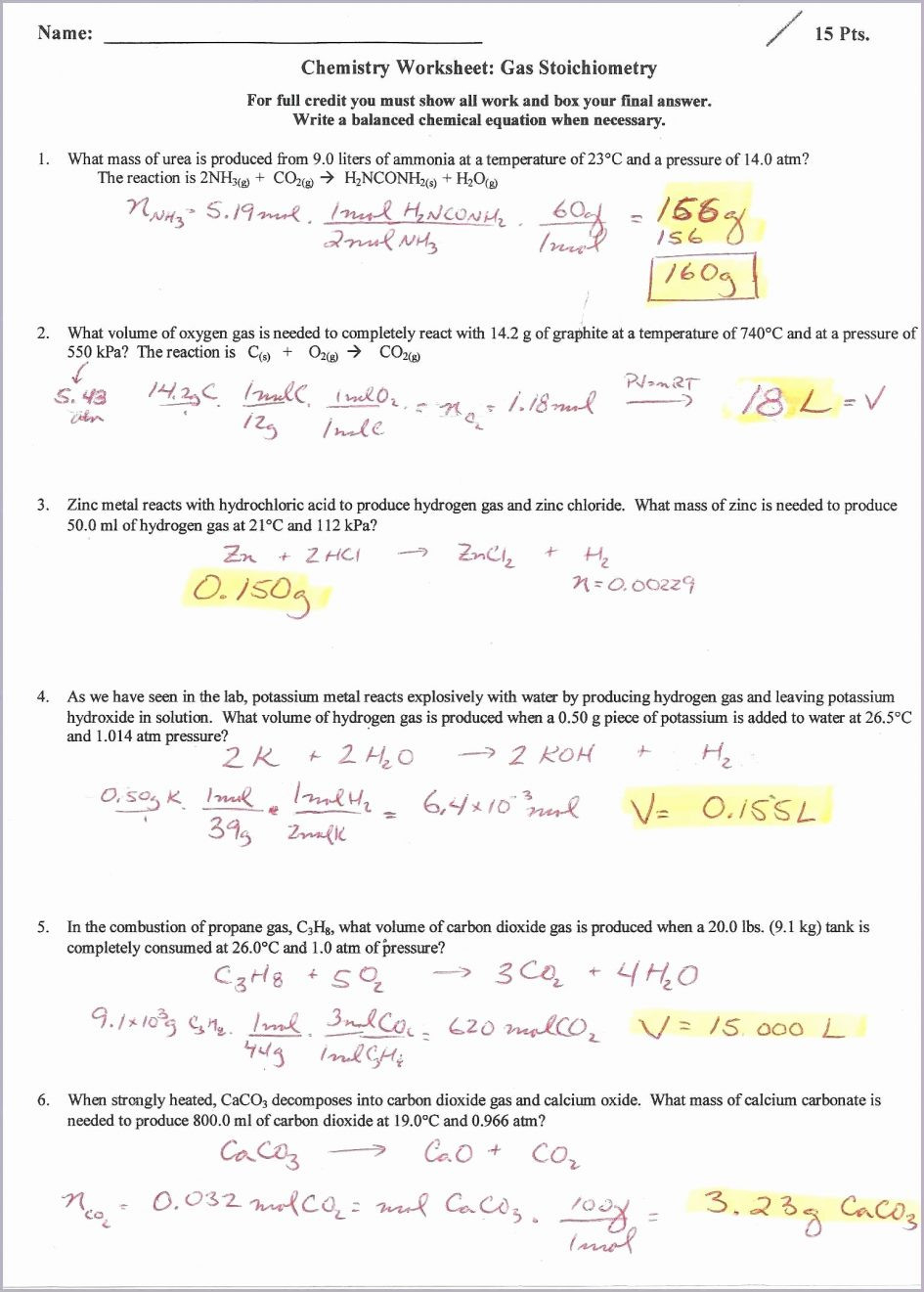
Gas Law Stoichiometry Worksheet - 2) how many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at stp? Web gas stoichiometry worksheet directions: How many grams of chlorine must react to produce 16 l of nitrogen gas at 1.2 atm and 23oc? Solve all the following gas law problems. Some of the worksheets for this concept are gas stoichiometry work name period gas, gas stoichiometry work name, gas stoichiometry work, ideal gas law practice work, gas stoichiometry work, , mixed gas laws work, ideal gas law work pv nrt. Use the gas laws we have learned to solve each of the following problems. The ideal gas law relates the four independent physical properties of a gas at any time. List the known quantities and solve the problem. How many milliliters of nitrogen can be made from 13 l of chlorine and 10.0 l of ammonia gas at stp? The chemical equations given are all balanced. Found worksheet you are looking for? Known mass c 2 h 5 oh = 25.21 g molar mass c 2 h 5 oh = 46.08 g/mol p = 728 mmhg t = 54 ∘ c = 327 k unknown volume co 2 =? How many milliliters of nitrogen can be made from 13 l of chlorine and 10.0 l of. A 45.6 ml sample of gas at 490 torr is compressed to a certain volume at 3 atm. Each of the chemical equations must first be balanced. When calcium carbonate is heated strongly, carbon dioxide gas is released according to the following equation: The chemical equations given are all balanced. Web gas law stoichiometry worksheet 1) for the reaction 2. Web use your knowledge of stoichiometry and the ideal gas law to solve the following problems. Web he determined that if certain gases that are products and reactions in a chemical reaction are measured at the same conditions, temperature and pressure, then the volume of gas consumed/produced is equal to the ratio between the gases or the ratio of the. Nn2 = (1.2atm) x (16l) Web gas stoichiometry worksheet name: Use the gas laws we have learned to solve each of the following problems. Each of the chemical equations must first be balanced. 1 l 10.0 l nh3 x 2 l nh3 = 5.00 l n2 n2 1 l 13 l cl2 x n2 3 l cl2 = 4.3 l. Web 1) if 4.00 moles of gasoline are burned, what volume of oxygen is needed if the pressure is 0.953 atm, and the temperature is 35.0°c? This is important for several reasons. The chemical equations given are all balanced. Use the gas laws we have learned to solve each of the following problems. The second half of the worksheet looks. Nn2 = (1.2atm) x (16l) List the known quantities and solve the problem. If water is added to magnesium nitride, ammonia gas is produced when the mixture is heated. Each of the chemical equations must first be balanced. Found worksheet you are looking for? A 50 cm3sample of nitrogen gas is collected at 101.3 kpa. When calcium carbonate is heated strongly, carbon dioxide gas is released according to the following equation: The chemical equations given are all balanced. If the volume is reduced to 5.0 cm3, and the temperatureremains constant, what will be the final pressure of the nitrogen? 1 l 10.0 l nh3. Web with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to calculate the stoichiometry of reactions involving gases, if the pressure and temperature are known. Web gas stoichiometry worksheet 1name: Web use your knowledge of stoichiometry and the ideal gas law to solve the following problems. Nn2. Solve all the following gas law problems. 1 l 10.0 l nh3 x 2 l nh3 = 5.00 l n2 n2 1 l 13 l cl2 x n2 3 l cl2 = 4.3 l n2 answer ________4.3 x 103 ml n2_________ b. Web gas stoichiometry worksheet name: Web use your knowledge of stoichiometry and the ideal gas law to solve. 2) how many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at stp? Web q1 using si units of kilograms, meters, and seconds with these fundamental equations, determine the combination of units that define the following: Then the ideal gas law is used to calculate the volume of co 2. Show. 2) how many liters of water can be made from 55 grams of oxygen gas and an excess of hydrogen at stp? Caco3(s) ⇒ cao(s) + co what volume of co 2(g), measured at stp, is produced if 21.5 g of caco 3(s) is heated? Web this product contains 15 pages of multiple choice questions on the ideal gas law, gas stoichiometry, gas reactions, calculating the molar mass of a gas, calculating the density of a gas solving for the pressure of a gas, solving for the volume of a gas, solving for the temperature of a gas or solvin. Web with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to calculate the stoichiometry of reactions involving gases, if the pressure and temperature are known. Web gas law stoichiometry worksheet 1) for the reaction 2 h2(g) + o2(g) ( 2 h2o(g), how many liters of water can be made from 5 l of oxygen gas and an excess of hydrogen at stp? Use the gas laws we have learned to solve each of the following problems. Web 1) if 4.00 moles of gasoline are burned, what volume of oxygen is needed if the pressure is 0.953 atm, and the temperature is 35.0°c? The second half of the worksheet looks at problems relating to gas densities, where the following The chemical equations given are all balanced. 1 l 10.0 l nh3 x 2 l nh3 = 5.00 l n2 n2 1 l 13 l cl2 x n2 3 l cl2 = 4.3 l n2 answer ________4.3 x 103 ml n2_________ b. Show all your work for credit. How many milliliters of nitrogen can be made from 13 l of chlorine and 10.0 l of ammonia gas at stp? When calcium carbonate is heated strongly, carbon dioxide gas is released according to the following equation: Then the ideal gas law is used to calculate the volume of co 2. A 50 cm3sample of nitrogen gas is collected at 101.3 kpa. Web gas stoichiometry worksheet name: Rated 4.86 out of 5, based on 7 reviews. Some of the worksheets for this concept are gas stoichiometry work name period gas, gas stoichiometry work name, gas stoichiometry work, ideal gas law practice work, gas stoichiometry work, , mixed gas laws work, ideal gas law work pv nrt. Gas laws and stoichiometry boyle’s law1. Web he determined that if certain gases that are products and reactions in a chemical reaction are measured at the same conditions, temperature and pressure, then the volume of gas consumed/produced is equal to the ratio between the gases or the ratio of the coefficients. Calculate the number of moles contained in 550.ml of carbon dioxide at stp. Web this product contains 15 pages of multiple choice questions on the ideal gas law, gas stoichiometry, gas reactions, calculating the molar mass of a gas, calculating the density of a gas solving for the pressure of a gas, solving for the volume of a gas, solving for the temperature of a gas or solvin. Web use your knowledge of stoichiometry and the ideal gas law to solve the following problems. Web gas stoichiometry worksheet directions: Consider the following unbalanced chemical equation for the combustion of propane: Known mass c 2 h 5 oh = 25.21 g molar mass c 2 h 5 oh = 46.08 g/mol p = 728 mmhg t = 54 ∘ c = 327 k unknown volume co 2 =? The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. Use the gas laws we have learned to solve each of the following problems. Web with the ideal gas law, we can use the relationship between the amounts of gases (in moles) and their volumes (in liters) to calculate the stoichiometry of reactions involving gases, if the pressure and temperature are known. How many milliliters of nitrogen can be made from 13 l of chlorine and 10.0 l of ammonia gas at stp? L the number of moles of carbon dioxide gas is first calculated by stoichiometry. When calcium carbonate is heated strongly, carbon dioxide gas is released according to the following equation: Web 1) if 4.00 moles of gasoline are burned, what volume of oxygen is needed if the pressure is 0.953 atm, and the temperature is 35.0°c? Web challenge your students with the ideal gas law and gas stoichiometry worksheets as classwork or homework. Web gas stoichiometry worksheet name: Then the ideal gas law is used to calculate the volume of co 2.Stoichiometry Test worksheet
Gas Law Stoichiometry Worksheet Ivuyteq
Solved Gas Stoichiometry Worksheet Name Solve all the
Stoichiometry Worksheet Answer Key —
FREE 9+ Sample Stoichiometry Worksheet Templates in MS Word PDF
11 Chemistry Gas Laws Worksheet /
Pin on ChemistryO
Worksheet Gas Stoichiometry Worksheet Ideal Gas Law —
Gas Stoichiometry Worksheet —
Background image of page 1 on Gas Stoichiometry Worksheet Science
How Many Grams Of Chlorine Must React To Produce 16 L Of Nitrogen Gas At 1.2 Atm And 23Oc?
Some Of The Worksheets For This Concept Are Gas Stoichiometry Work Name Period Gas, Gas Stoichiometry Work Name, Gas Stoichiometry Work, Ideal Gas Law Practice Work, Gas Stoichiometry Work, , Mixed Gas Laws Work, Ideal Gas Law Work Pv Nrt.
Web Gas Law Stoichiometry Worksheet Solving Gas Law Problems That Require Stoichiometry 1) For The Reaction 2 H2(G) + O2(G) ( 2 H2O(G), How Many Liters Of Water Can Be Made From 5 L Of Oxygen Gas And An Excess Of Hydrogen At Stp?
Web He Determined That If Certain Gases That Are Products And Reactions In A Chemical Reaction Are Measured At The Same Conditions, Temperature And Pressure, Then The Volume Of Gas Consumed/Produced Is Equal To The Ratio Between The Gases Or The Ratio Of The Coefficients.
Related Post: