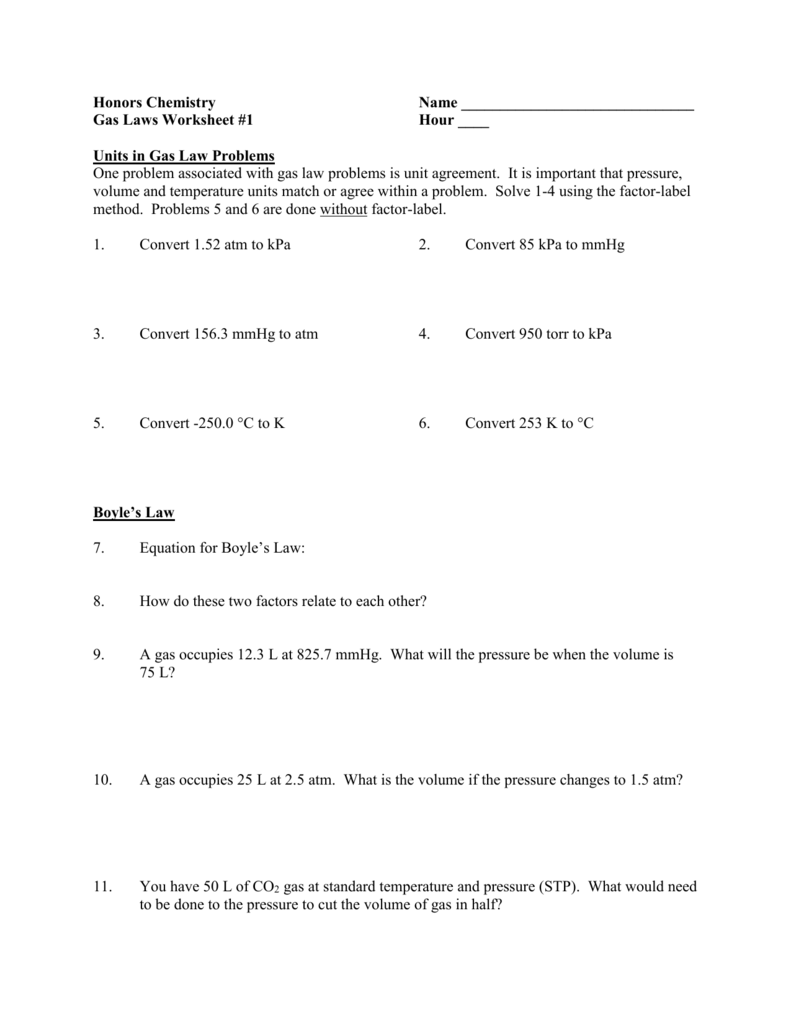
Gas Laws Worksheet 1
Gas Laws Worksheet 1 - The correct answer is given in. If 5.0 moles of o2 and 3.0 moles of n2 are. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. A gas sample contained in a cylinder equipped with a moveable piston. What volume will the gas. Web a good worksheet for teaching the students when to use the ideal gas law and when to use the combined gas law! Atm = 760 mm hg = 101 kpa= 760.0 torr. Gas laws worksheet combined if 22.5 of nitrogen at 748 mm hg are compressed skip to document ask an expert sign inregister sign inregister. A gas has a volume of 5 liters at 3 atm. What will be the volume at 1 atm? What would be the volume of the balloon in the. Web for this to be true, temperature and amount of gas must remain constant this can be expressed mathematically as pv = k where k is a constant that depends on how much. To expand the volume to 7500 ml, what the new pressure (. A sample of gas is. Using the gas law equations: A sample of 0.50 moles of gas is placed in a container of volume of 2.5 l. A gas sample contained in a cylinder equipped with a moveable piston. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. Web gas laws worksheet #1 1. A gas has a volume of 5 liters at 3 atm. What volume of gas is needed. Web mixed gas laws worksheet how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? Web gas laws 1 (worksheet) q1. A 2.5 l fire extinguisher has an internal pressure of 5.5 atm. Calculate the pressure if the temperature is changed to 127 c while the volume remains constant. A 2.5 l fire extinguisher has an internal pressure of 5.5 atm. If 5.0 moles of o2 and 3.0 moles of n2 are. What is the pressure of the gas in torr. The correct answer is given in. Web mixed gas laws worksheet how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? If 5.0 moles of o2 and 3.0 moles of n2 are. A 2.5 l fire extinguisher has an internal pressure of 5.5 atm. A gas sample contained in a cylinder equipped with a moveable piston.. Web a good worksheet for teaching the students when to use the ideal gas law and when to use the combined gas law! What is the pressure of the gas in torr. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. Calculate the pressure if the temperature is changed to 127. Calculate the pressure if the temperature is changed to 127 c while the volume remains constant. A gas has a volume of 5 liters at 3 atm. Atm = 760 mm hg = 101 kpa= 760.0 torr. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. Convert 1.8 atm into kpa. To expand the volume to 7500 ml, what the new pressure (. What volume of gas is needed. A sample of gas has a volume of 215 cm3 at 23.5 °c and 84.6 kpa. A sample of gas has a pressure of 100.0 torr and 27.0 c. What will be the volume at 1 atm? If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant. If 5.0 moles of o2 and 3.0 moles of n2 are. A sample of gas is characterized by specifying its amount, its. What will be the volume at 1 atm? A gas sample contained in a cylinder equipped with a moveable piston. A balloon was blown up to a volume of 2.15 l on a day when atmospheric pressure was 100.3 kpa. A sample of gas is placed. What volume of gas is needed. What will be the volume at 1 atm? A gas sample contained in a cylinder equipped with a moveable piston. A sample of gas is placed. A gas initially at stp is changed to 248 k. A sample of gas has a volume of 215 cm3 at 23.5 °c and 84.6 kpa. Act fast, we're transitioning soon! If 5.0 moles of o2 and 3.0 moles of n2 are. What will be the volume at 1 atm? Web gas laws 1 (worksheet) q1. Using the gas law equations: A sample of 0.50 moles of gas is placed in a container of volume of 2.5 l. Web a gas has a volume of 460 ml at 500. What would be the volume of the balloon in the. 1 atm = 760.0 mm hg = 101.3 kpa. Calculate the final pressure of the gas. Atm = 760 mm hg = 101 kpa= 760.0 torr. The correct answer is given in. A sample of gas is characterized by specifying its amount, its. Web a good worksheet for teaching the students when to use the ideal gas law and when to use the combined gas law! Convert 25.9 psi into atm. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. The correct answer is given in. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. Web for this to be true, temperature and amount of gas must remain constant this can be expressed mathematically as pv = k where k is a constant that depends on how much. A sample of gas has a volume of 215 cm3 at 23.5 °c and 84.6 kpa. A gas has a volume of 5 liters at 3 atm. What will be the volume at 1 atm? Act fast, we're transitioning soon! If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant. If 5.0 moles of o2 and 3.0 moles of n2 are. A gas initially at stp is changed to 248 k. What is the pressure of the gas in torr. Calculate the final pressure of the gas. 1 atm = 760.0 mm hg = 101.3 kpa. Convert 1.8 atm into kpa. Gas laws worksheet combined if 22.5 of nitrogen at 748 mm hg are compressed skip to document ask an expert sign inregister sign inregister. A 2.5 l fire extinguisher has an internal pressure of 5.5 atm.Gas Laws Worksheet 1
Gas Law Worksheet 1
Gas Law Worksheet 1
Gas Laws Worksheet 1 Focus Wiring
Gas Laws Worksheet 1
Ideal Gas Law Worksheet Answers Questions Answer Zac Sheet
Boyles And Charles Law Worksheet defendusinbattleblog Worksheet
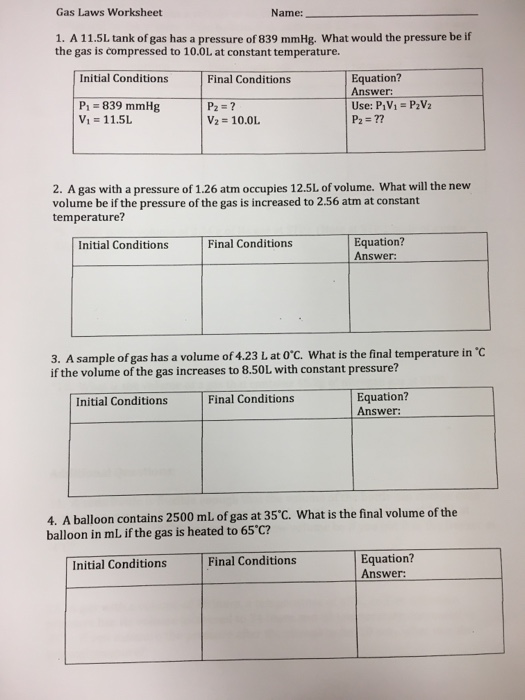
Solved Gas Laws Worksheet Name 1. A 11.5L tank of gas has a
11 Chemistry Gas Laws Worksheet /
Gas Laws Worksheet 1
Calculate The Pressure If The Temperature Is Changed To 127 C While The Volume Remains Constant.
A Gas Sample Contained In A Cylinder Equipped With A Moveable Piston.
A Gas Occupies A Volume Of 50.0 Ml At 27 C And 630 Mmhg.
A Sample Of Gas Is Placed.
Related Post: