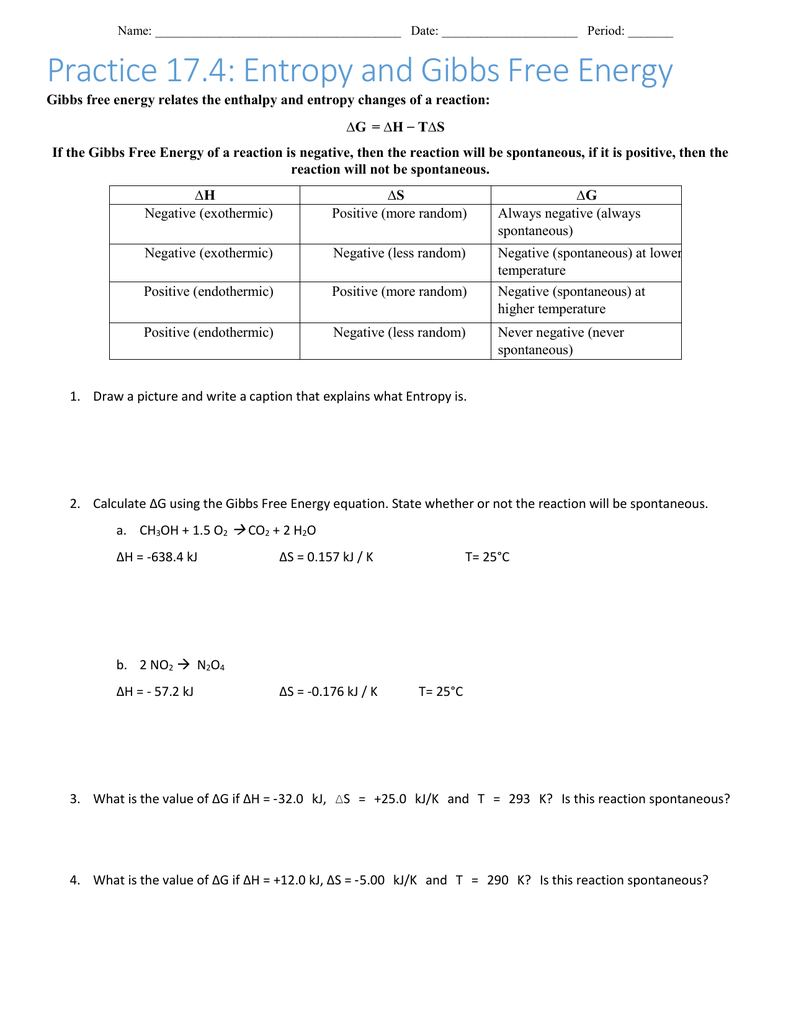
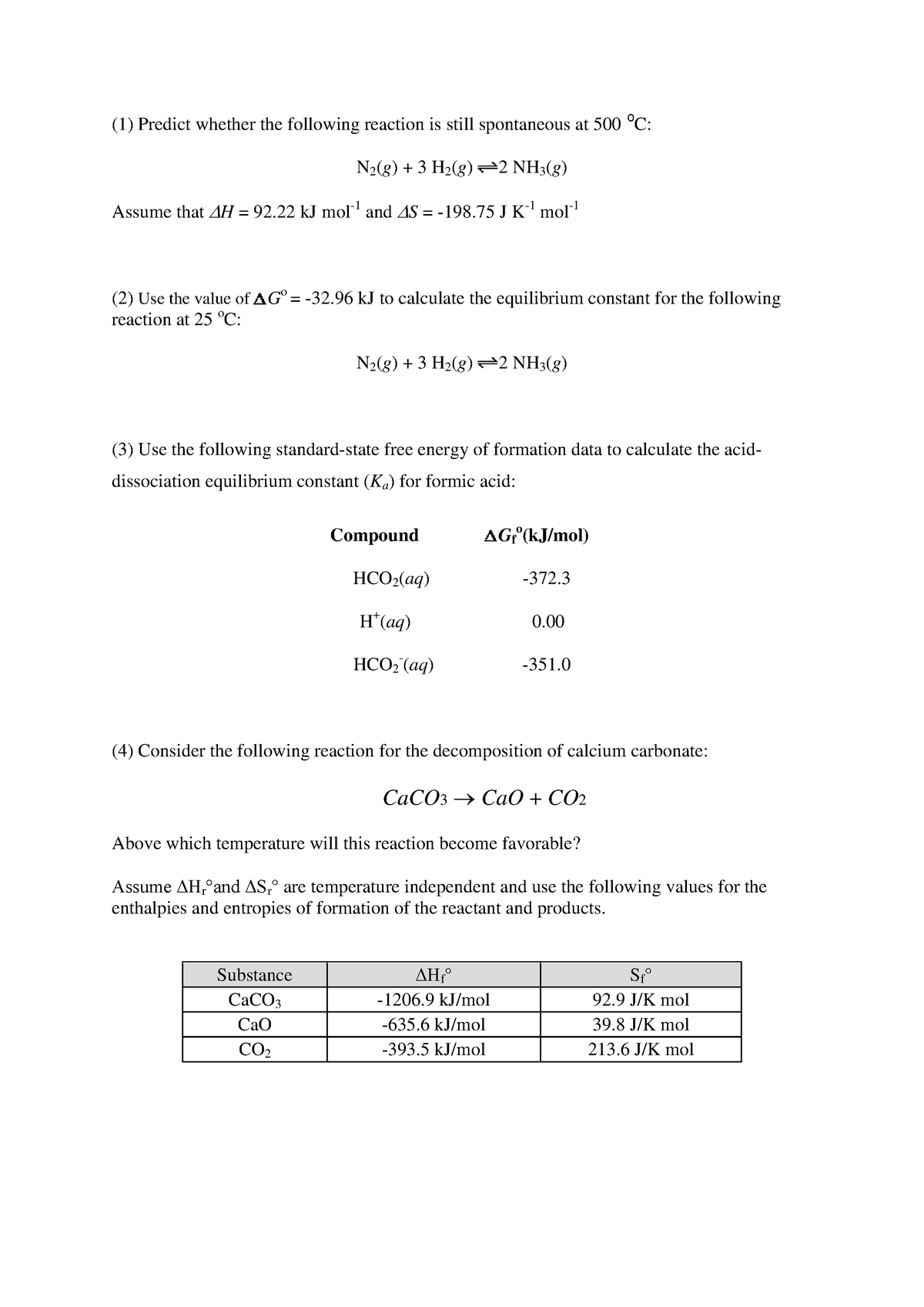
Gibbs Free Energy Worksheet Answers
Gibbs Free Energy Worksheet Answers - Is spontaneous at 300 k and. You'll be asked to define gibbs free energy and. A.) it is greater than zero. The standard free energy change for a reaction may also be. Web entropy and gibbs free energy entropy 1. Web up to 24% cash back gibb’s free energy problems worksheet the hydrogenation of ethene gas under standard conditions (t = 298.15 k) shows a decrease in disorder (δs˚ =. Web c2h6(g) h2(g) + c2h4(g) answer: Calculate the change in entropy of a large vat of molten copper when 50 j of energy is removed reversible from it as heat at 1100 °c. The reaction is nonspontaneous ( not spontaneous) at 25 °c. Paul andersen attempts to explain gibbs free energy. Calculate the change in entropy of a large vat of molten copper when 50 j of energy is removed reversible from it as heat at 1100 °c. Web up to 24% cash back gibb’s free energy problems worksheet the hydrogenation of ethene gas under standard conditions (t = 298.15 k) shows a decrease in disorder (δs˚ =. Web the gibbs. ∆h (kj) t∆s (kj) a). A.) it is greater than zero. If the reaction quotient (q) is greater than the equilibrium constant (k), what is true about the gibbs free energy? B.) it is less than zero. You'll be asked to define gibbs free energy and. Web entropy and gibbs free energy entropy 1. A) the sign of entropy would be positive. The standard free energy change for a reaction may also be. Web study with quizlet and memorize flashcards containing terms like gibb free energy is equal to, formula for gibbs free energy, if gibbs free energy is less than 0 then the reaction. If. He begins by using three spontaneous reactions to explain how a change in enthalpy,. Web entropy and gibbs free energy entropy 1. The standard free energy change for a reaction may also be. Web the gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature. Free energy and the equilibrium constant. You'll be asked to define gibbs free energy and. Calculate the change in entropy of a large vat of molten copper when 50 j of energy is removed reversible from it as heat at 1100 °c. Web c2h6(g) h2(g) + c2h4(g) answer: Since we are looking at temperatures, in order for ∆s to. A) the sign of entropy would be positive. Mathematical formula for this value is: Web for each reaction, calculate the value of gibb’s free energy (∆g) and predict whether the reaction is spontaneous or not spontaneous in the forward direction. Ice melting at 25°c heat flowing from a hot to a cold object an iron tool rusting 1 only 2. Web up to 24% cash back gibb’s free energy problems worksheet the hydrogenation of ethene gas under standard conditions (t = 298.15 k) shows a decrease in disorder (δs˚ =. The reaction is nonspontaneous ( not spontaneous) at 25 °c. Ice melting at 25°c heat flowing from a hot to a cold object an iron tool rusting 1 only 2. Going from a solid to gas b) gibbs free energy would be negative. Web gibbs free energy name for a reaction to be spontaneous, the sign of ag (gibbs free energy) must be negative. ∆h (kj) t∆s (kj) a). Since we are looking at temperatures, in order for ∆s to. Web exercise 18.1a which of the following is a spontaneous. Going from a solid to gas b) gibbs free energy would be negative. Web study with quizlet and memorize flashcards containing terms like gibb free energy is equal to, formula for gibbs free energy, if gibbs free energy is less than 0 then the reaction. Is spontaneous at 300 k and. Ice melting at 25°c heat flowing from a hot. You'll be asked to define gibbs free energy and. Web study with quizlet and memorize flashcards containing terms like gibb free energy is equal to, formula for gibbs free energy, if gibbs free energy is less than 0 then the reaction. Calculate the change in entropy of a large vat of molten copper when 50 j of energy is removed. You'll be asked to define gibbs free energy and. Since we are looking at temperatures, in order for ∆s to. Web gibbs free energy name for a reaction to be spontaneous, the sign of ag (gibbs free energy) must be negative. Web study with quizlet and memorize flashcards containing terms like gibb free energy is equal to, formula for gibbs free energy, if gibbs free energy is less than 0 then the reaction. Web gibbs free energy. Free energy and the equilibrium constant. Web understand the significance of the thermodynamic functions of entropy and gibbs free energy understand the relationships between both entropy and gibbs free. Mathematical formula for this value is: Going from a solid to gas b) gibbs free energy would be negative. Web up to 24% cash back gibb’s free energy problems worksheet the hydrogenation of ethene gas under standard conditions (t = 298.15 k) shows a decrease in disorder (δs˚ =. The standard free energy change for a reaction may also be. Web exercise 18.1a which of the following is a spontaneous process? The reaction has a positive entropy and a negative enthalpy. A.) it is greater than zero. Web the gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system. Web c2h6(g) h2(g) + c2h4(g) answer: A) the sign of entropy would be positive. If the reaction quotient (q) is greater than the equilibrium constant (k), what is true about the gibbs free energy? Is spontaneous at 300 k and. The reaction is nonspontaneous ( not spontaneous) at 25 °c. A) the sign of entropy would be positive. Calculate the change in entropy of a large vat of molten copper when 50 j of energy is removed reversible from it as heat at 1100 °c. Is spontaneous at 300 k and. Since we are looking at temperatures, in order for ∆s to. Web for each reaction, calculate the value of gibb’s free energy (∆g) and predict whether the reaction is spontaneous or not spontaneous in the forward direction. You'll be asked to define gibbs free energy and. ∆h (kj) t∆s (kj) a). Web exercise 18.1a which of the following is a spontaneous process? Free energy and the equilibrium constant. Web gibbs free energy name for a reaction to be spontaneous, the sign of ag (gibbs free energy) must be negative. The reaction has a positive entropy and a negative enthalpy. Paul andersen attempts to explain gibbs free energy. If the reaction quotient (q) is greater than the equilibrium constant (k), what is true about the gibbs free energy? Mathematical formula for this value is: Web study with quizlet and memorize flashcards containing terms like gibb free energy is equal to, formula for gibbs free energy, if gibbs free energy is less than 0 then the reaction. Web the gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system.Gibbs Free Energy Worksheet Answer Key worksheet
Gibbs Free Energy Worksheet Answers Study Finder
√ 20 Gibbs Free Energy Worksheet Simple Template Design
30 Gibbs Free Energy Worksheet Education Template
Gibbs Free Energy Worksheet Answers worksheet
Gibbs Free Energy Worksheet
Gibbs Free Energy Worksheet Worksheet List
√ 20 Gibbs Free Energy Worksheet Simple Template Design
Gibbs Free Energy Worksheet Answer Key worksheet
Gibbs Free Energy Worksheet Answers Study Finder
The Standard Free Energy Change For A Reaction May Also Be.
Web Gibbs Free Energy.
Web Entropy And Gibbs Free Energy Entropy 1.
B.) It Is Less Than Zero.
Related Post: