Graham's Law Of Effusion Worksheet Answers
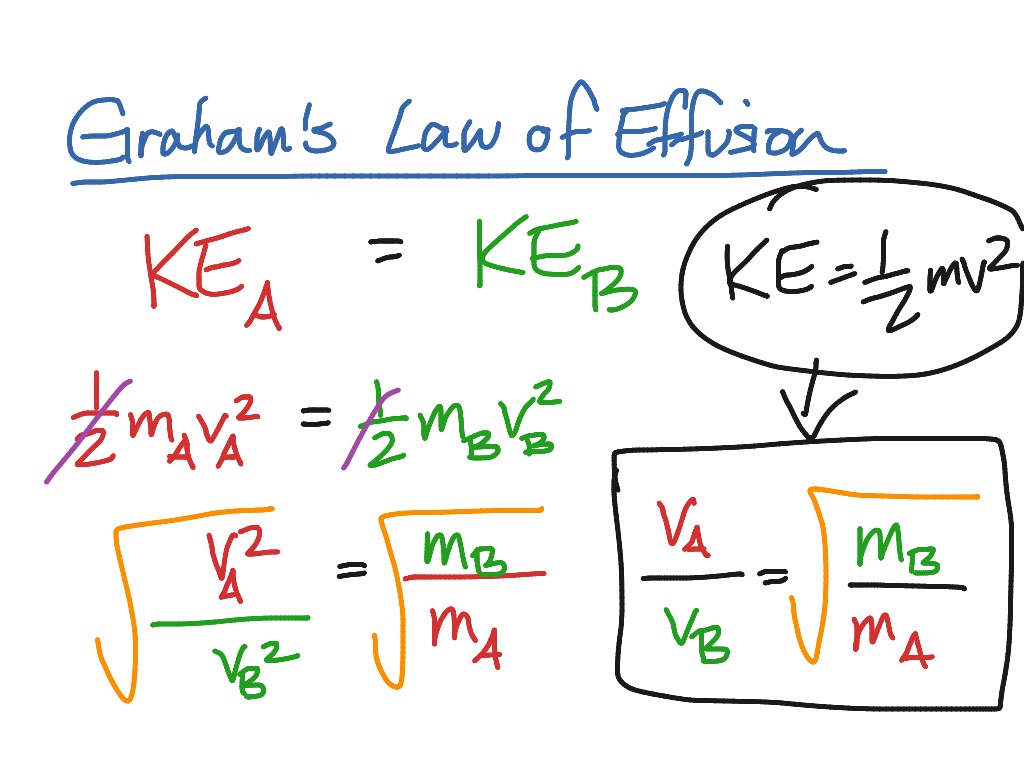
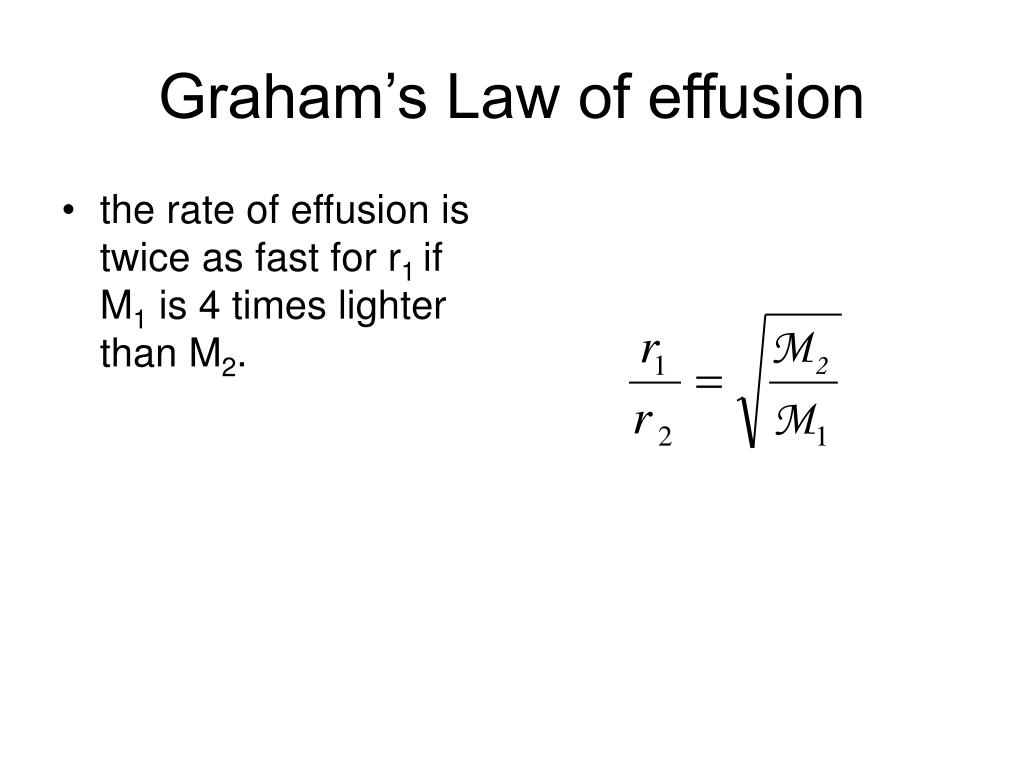
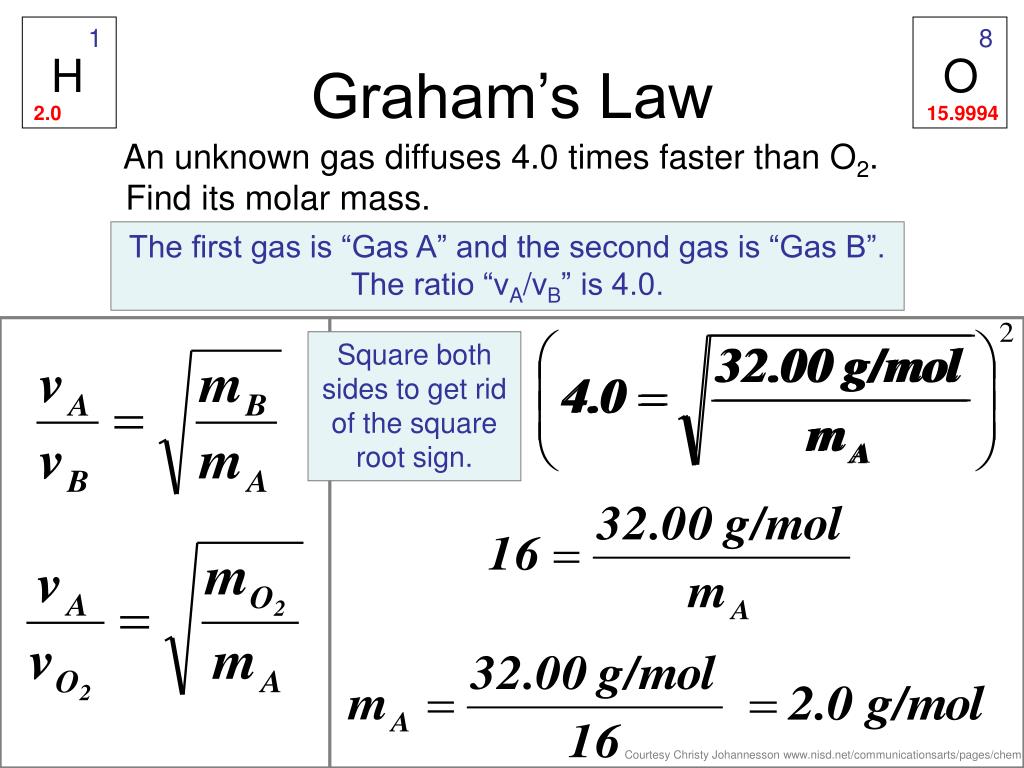
Graham's Law Of Effusion Worksheet Answers - If neon gas travels at 400 m/s at a given temperature, calculate the. Web graham's law states that the rate of diffusion or of effusion of a gas is inversely proportional to the square root of its molecular weight. Hence we understand that the ratio of diffusion rates of the given gases should be 1/2.92. Under conditions in which the density of carbon dioxide is 1.96 g/l and that of nitrogen is 1.25 g/l, which gas will effuse. Web 4.5 satisfied 143 votes what makes the grahams law worksheet legally valid? = 2√ 1 must show work for each problem: This allows for determining the molar mass of. Thus, if the molecular weight of. What will be the ratio of the. Web this worksheet covers graham's law of effusion. Because the society takes a step away from office working conditions, the execution of paperwork. Web this worksheet covers graham's law of effusion. = 2√ 1 must show work for each problem: Web graham's law states that the rate of diffusion or of effusion of a gas is inversely proportional to the square root of its molecular weight. Under conditions. This allows for determining the molar mass of. _____ graham’s law states that a gas will effuse at a rate that is inversely proportional to the square root of its. What will be the ratio of the. Web one application of graham’s law is the formula obtained for two gases correlating their molar masses and effusion rates: Web diffusion is. Because the society takes a step away from office working conditions, the execution of paperwork. Effusion refers to the movement of gas particles through a small hole. Thus, if the molecular weight of. = 2√ 1 must show work for each problem: If neon gas travels at 400 m/s at a given temperature, calculate the. If neon gas travels at 400 m/s at a given temperature, calculate the. Worksheets are chemistry grahams law, graham s law, lab grahams law. Effusion refers to the movement of gas particles through a small hole. Web diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Under conditions in which the density of carbon dioxide. Web graham's law states that the rate of diffusion or of effusion of a gas is inversely proportional to the square root of its molecular weight. Web graham’s law of effusion and diffusion name: Because the society takes a step away from office working conditions, the execution of paperwork. If neon gas travels at 400 m/s at a given temperature,. Web 2√ 1 diffusion √ 2= ; This allows for determining the molar mass of. Worksheets are chemistry grahams law, grahams law of effusion, daltons law work, example exercise henrys law, gas. Some of the sheets for this concept are the grahams law effusing,, the chemistry grahams act,. Worksheets are chemistry grahams law, graham s law, lab grahams law. Effusion refers to the movement of gas particles through a small hole. Hence we understand that the ratio of diffusion rates of the given gases should be 1/2.92. Web starting with the definition of rate of effusion and graham’s finding relating rate and molar mass, show how to derive the graham’s law equation, relating the relative rates of. Web 4.5. What will be the ratio of the. We know that the diffusion rate is 2.92 times of ammonia; _____ graham’s law states that a gas will effuse at a rate that is inversely proportional to the square root of its. Web 2√ 1 diffusion √ 2= ; Under conditions in which the density of carbon dioxide is 1.96 g/l and. Thus, if the molecular weight of. Under conditions in which the density of carbon dioxide is 1.96 g/l and that of nitrogen is 1.25 g/l, which gas will effuse. Web starting with the definition of rate of effusion and graham’s finding relating rate and molar mass, show how to derive the graham’s law equation, relating the relative rates of. Some. Web grahams law of effusion. Under conditions in which the density of carbon dioxide is 1.96 g/l and that of nitrogen is 1.25 g/l, which gas will effuse. Web one application of graham’s law is the formula obtained for two gases correlating their molar masses and effusion rates: Web graham’s law of effusion and diffusion name: Some of the sheets. What will be the ratio of the. Web graham’s law is an empirical relationship that states that the ratio of the rates of diffusion or effusion of two gases is the square root of the inverse ratio of their molar masses. These 16 questions and problems will ensure that your chemistry or physical science students completely understand the. Web 2√ 1 diffusion √ 2= ; = 2√ 1 must show work for each problem: Under conditions in which the density of carbon dioxide is 1.96 g/l and that of nitrogen is 1.25 g/l, which gas will effuse. We know that the diffusion rate is 2.92 times of ammonia; Worksheets are chemistry grahams law, graham s law, lab grahams law. Web 4.5 satisfied 143 votes what makes the grahams law worksheet legally valid? Web up to 24% cash back 1. Thus, if the molecular weight of. Effusion refers to the movement of gas particles through a small hole. Under conditions in which the density of carbon dioxide is 1.96 g/l and that of nitrogen gas is 1.25 g/l, which gas will effuse more rapidly? Because the society takes a step away from office working conditions, the execution of paperwork. Web this worksheet covers graham's law of effusion. Web diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. If neon gas travels at 400 m/s at a given temperature, calculate the. Web grahams law of effusion. Worksheets are chemistry grahams law, grahams law of effusion, daltons law work, example exercise henrys law, gas. Web this practice problem worksheet can be used for classwork, homework, test review, or quiz.students will define diffusion and effusion.students will state graham's law and. Web graham’s law is an empirical relationship that states that the ratio of the rates of diffusion or effusion of two gases is the square root of the inverse ratio of their molar masses. Web up to 24% cash back 1. Worksheets are chemistry grahams law, grahams law of effusion, daltons law work, example exercise henrys law, gas. Web one application of graham’s law is the formula obtained for two gases correlating their molar masses and effusion rates: Worksheets are chemistry grahams law, graham s law, lab grahams law. _____ graham’s law states that a gas will effuse at a rate that is inversely proportional to the square root of its. Because the society takes a step away from office working conditions, the execution of paperwork. Some of the sheets for this concept are the grahams law effusing,, the chemistry grahams act,. Under conditions in which the density of carbon dioxide is 1.96 g/l and that of nitrogen gas is 1.25 g/l, which gas will effuse more rapidly? Web 2√ 1 diffusion √ 2= ; Web graham's law states that the rate of diffusion or of effusion of a gas is inversely proportional to the square root of its molecular weight. Web this practice problem worksheet can be used for classwork, homework, test review, or quiz.students will define diffusion and effusion.students will state graham's law and. Web diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Effusion refers to the movement of gas particles through a small hole. Thus, if the molecular weight of. These 16 questions and problems will ensure that your chemistry or physical science students completely understand the.Graham's Law Of Effusion Worksheet Answers
Graham's Law Of Effusion Worksheet Answer Key Key Worksheet
graham's law worksheet
Graham's Law Of Effusion Worksheet
Graham's Law Of Effusion Worksheet Answers
Chapter 10 Lecture Gases
PPT Gases and gas laws PowerPoint Presentation, free download ID619593
Quiz & Worksheet Graham's Law for Diffusion and Effusion
Gases 6 Graham's Law of Effusion .Gases 6 Graham's Law of Effusion
Graham's Law Of Effusion Worksheet Answers
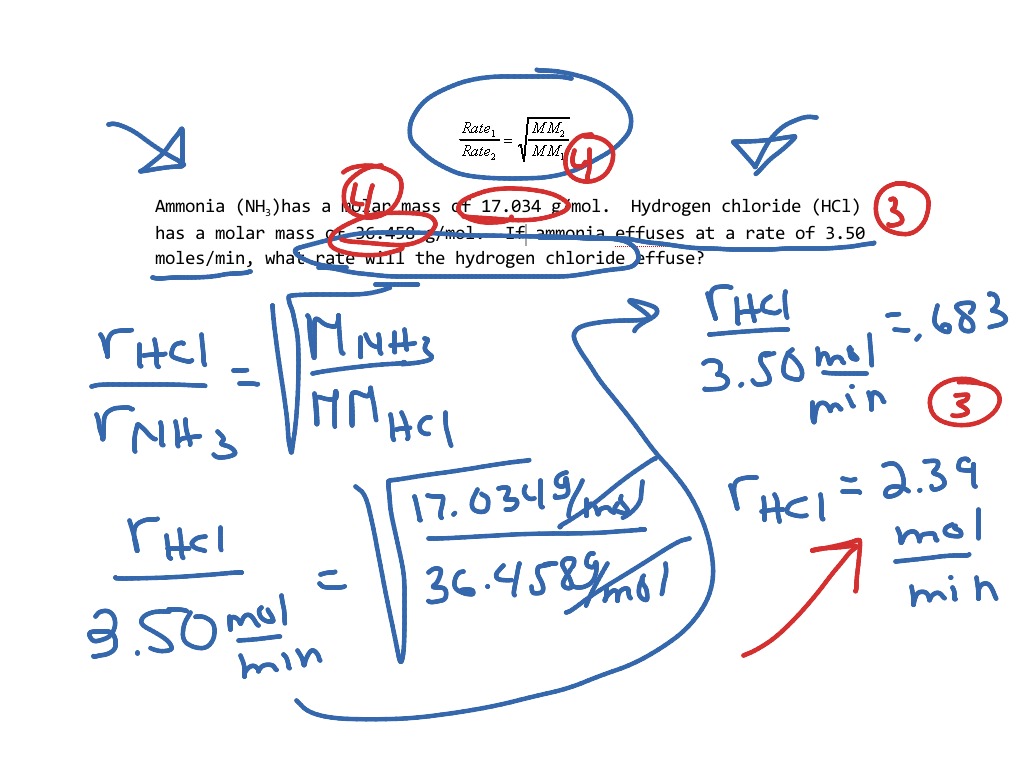
= 2√ 1 Must Show Work For Each Problem:
Web This Worksheet Covers Graham's Law Of Effusion.
We Know That The Diffusion Rate Is 2.92 Times Of Ammonia;
Under Conditions In Which The Density Of Carbon Dioxide Is 1.96 G/L And That Of Nitrogen Is 1.25 G/L, Which Gas Will Effuse.
Related Post: