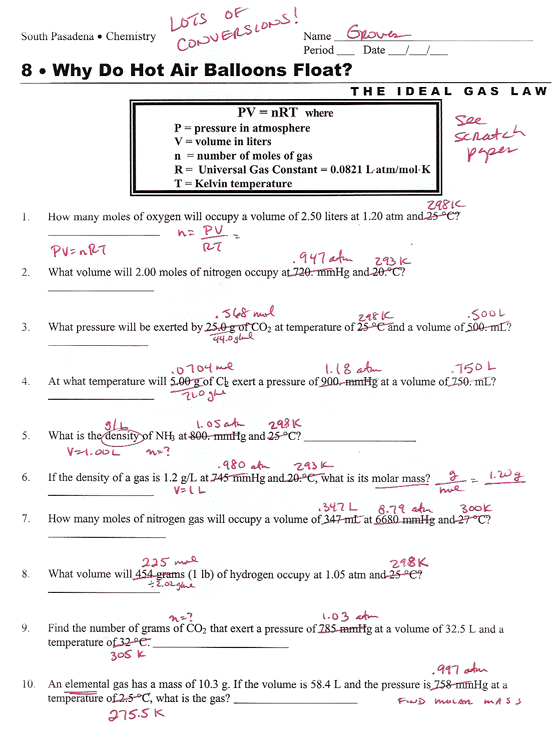
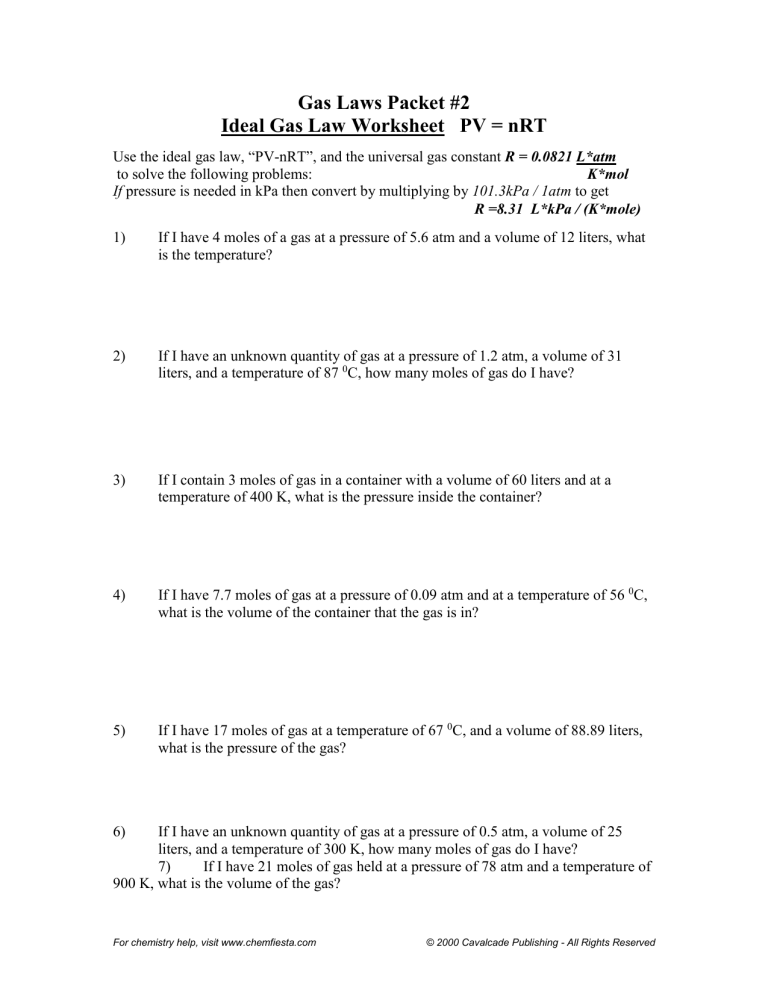
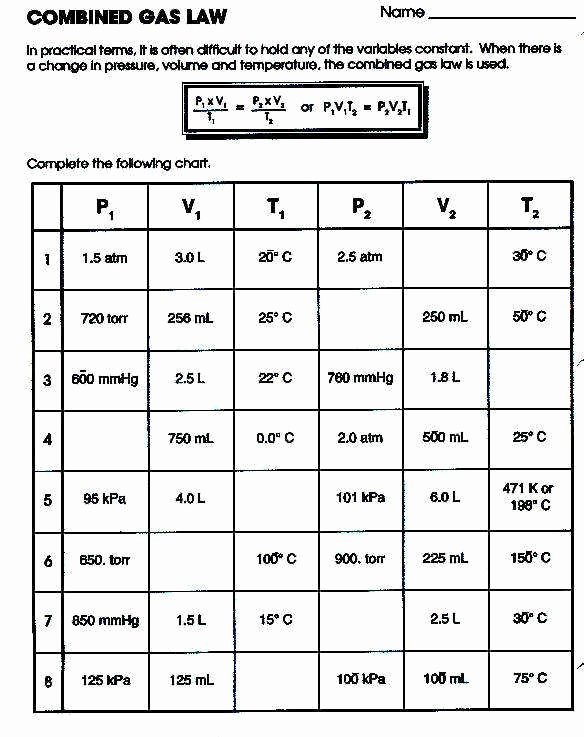
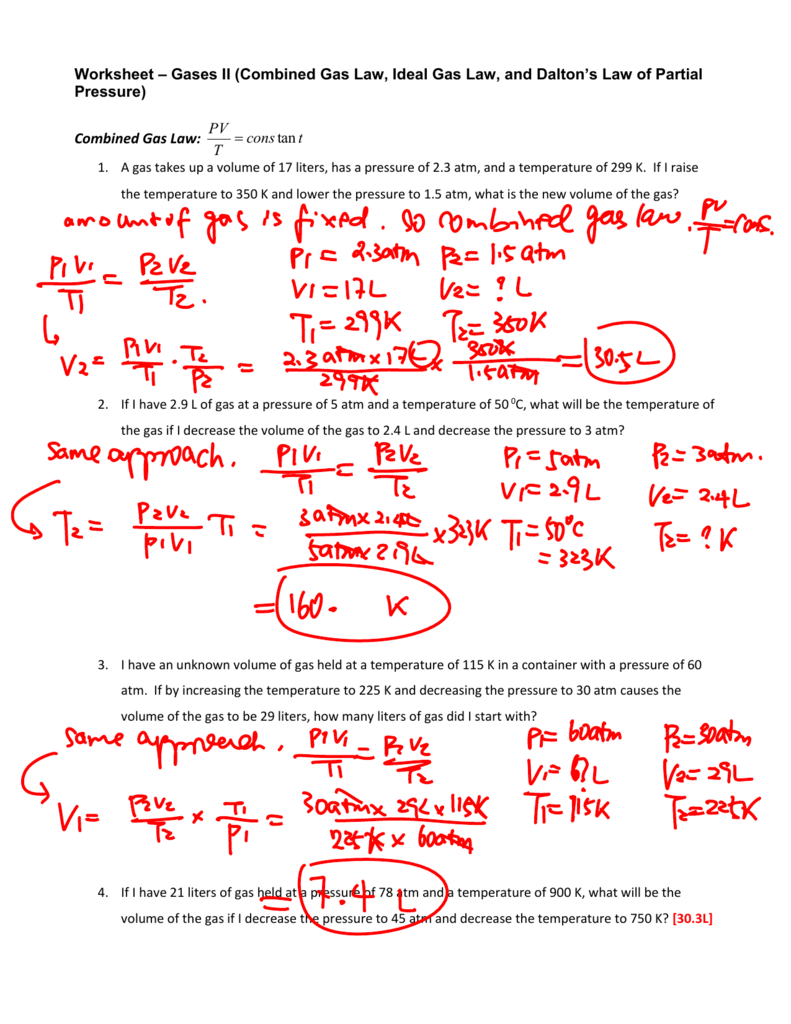
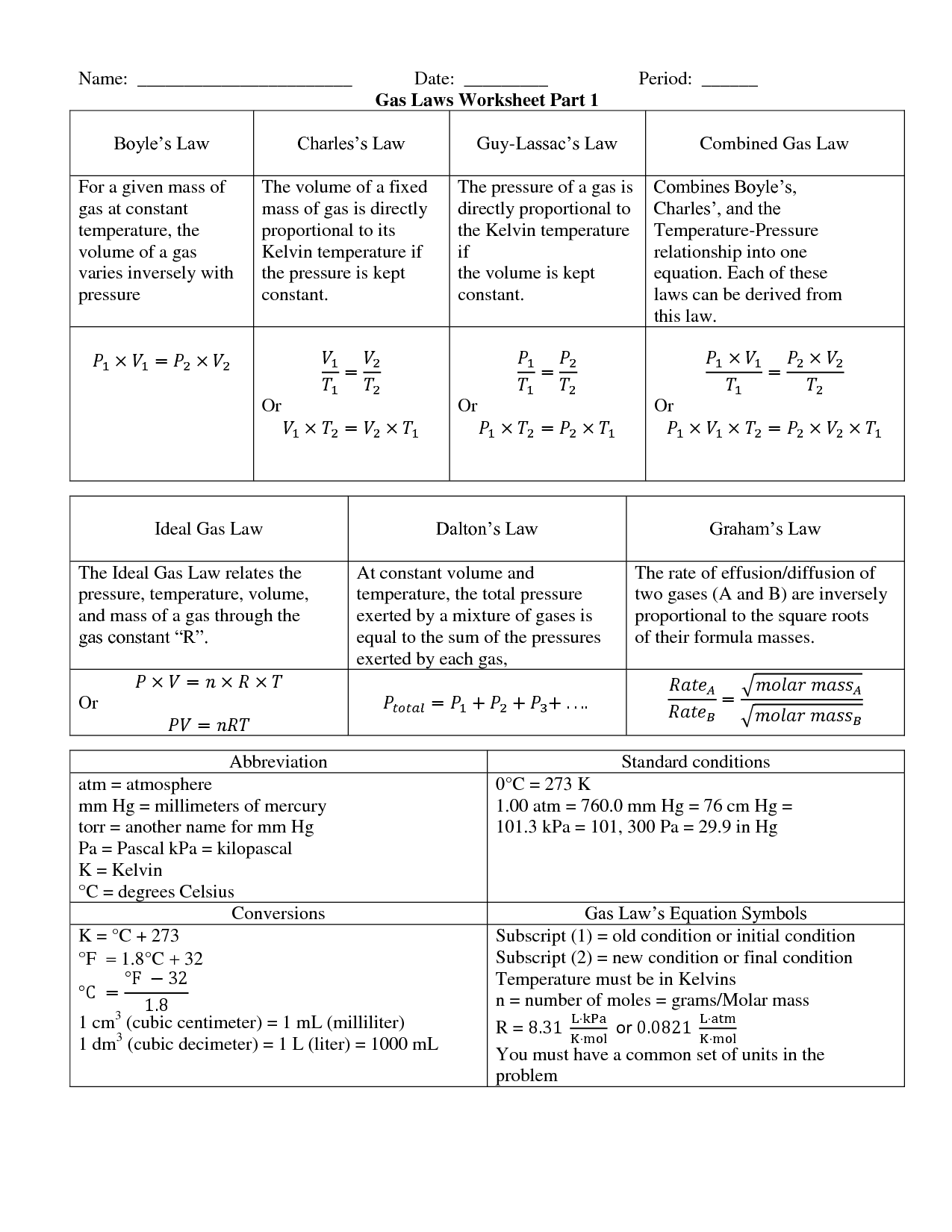
Ideal Gas Law Packet Worksheet Answers
Ideal Gas Law Packet Worksheet Answers - The column on the right is so you can practice quickly identifying which gas law is being. The ideal gas law name: Real gases behave like ideal gases except at very high temperatures. The ideal gas law states that pv = nrt , where p is the pressure of a gas, v is the volume of the gas, n is the number of moles. 2311 l 2311 l 5) if i have 17. On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships between the number of moles, the mass, and the. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant. For any sample of gas under ideal conditions, the relationship between the amount of gas in moles (n) and its temperature, pressure, and volume is. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of. Web write your answer on the line provided. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant. The ideal gas law states that pv = nrt , where p is the. The column on the right is so you can practice quickly identifying which gas law is being. Web the ideal gas law investigates the relationship between pressure, volume, temperature, and moles of a gas. How are pressure, volume, and temperature related? Web use your knowledge of the ideal and combined gas laws to solve the following problems. Identify an ideal. What have these simulations and the gas laws shown us? 2311 l 2311 l 5) if i have 17. The column on the right is so you can practice quickly identifying which gas law is being. Web write your answer on the line provided. State what the 'n' represents in the ideal gas equation. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. Demo/lab 7 day 6 lesson plan: Web some answers provided at the end of the question. A sample of \ce {h2} (g) hx 2(g) is contained in a cylinder with a moveable piston at an initial pressure of p_1 p 1. For. 2311 l 2311 l 5) if i have 17. Web quiz & worksheet goals. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of. The ideal gas law name: A sample of \ce {h2} (g) hx 2(g) is contained in a. Web solutions to the ideal gas law practice worksheet: The ideal gas law states that pv = nrt , where p is the pressure of a gas, v is the volume of the gas, n is the number of moles. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant. The ideal gas. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. The ideal gas law name: The gas constant, r, is equal to 0.0821 when. 1 atm = 760.0 mm hg = 101.3 kpa. 2311 l 2311 l 5) if i have 17. Web quiz & worksheet goals. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of. State what the 'n' represents in the ideal gas equation. How are pressure, volume, and temperature related? Demo/lab 7 day 6 lesson plan: If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of. Web solutions to the ideal gas law practice worksheet: The column on the right is so you can practice quickly identifying which gas law is being. Web ideal gas law. Web ideal gas law practice worksheet solve. Web ideal gas law practice worksheet solve the following problems using the ideal gas law: The ideal gas law name: When you take this quiz, you'll need to be able to: A sample of \ce {h2} (g) hx 2(g) is contained in a cylinder with a moveable piston at an initial pressure of p_1 p 1. The gas constant, r,. What have these simulations and the gas laws shown us? A sample of \ce {h2} (g) hx 2(g) is contained in a cylinder with a moveable piston at an initial pressure of p_1 p 1. Real gases behave like ideal gases except at very high temperatures. The gas constant, r, is equal to 0.0821 when. Web quiz & worksheet goals. For any sample of gas under ideal conditions, the relationship between the amount of gas in moles (n) and its temperature, pressure, and volume is. Solve each of the following problems. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant. Web solutions to the ideal gas law practice worksheet: Demo/lab 7 day 6 lesson plan: Web some answers provided at the end of the question. Identify an ideal gas condition. Web ideal gas law. Web write your answer on the line provided. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of. 64 atm 4) if i have 7.7 moles of gas at a pressure of 0.09 atm and at a temperature of 560c, what is the volume of the container that the gas is in? State what the 'n' represents in the ideal gas equation. How are pressure, volume, and temperature related? The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of. Web the ideal gas law investigates the relationship between pressure, volume, temperature, and moles of a gas. What have these simulations and the gas laws shown us? Solve each of the following problems. A sample of \ce {h2} (g) hx 2(g) is contained in a cylinder with a moveable piston at an initial pressure of p_1 p 1. The column on the right is so you can practice quickly identifying which gas law is being. Web use your knowledge of the ideal and combined gas laws to solve the following problems. How are pressure, volume, and temperature related? Web ideal gas law. 64 atm 4) if i have 7.7 moles of gas at a pressure of 0.09 atm and at a temperature of 560c, what is the volume of the container that the gas is in? The ideal gas law states that pv = nrt , where p is the pressure of a gas, v is the volume of the gas, n is the number of moles. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of. Web solutions to the ideal gas law practice worksheet: State what the 'n' represents in the ideal gas equation. 1 atm = 760.0 mm hg = 101.3 kpa. Web some answers provided at the end of the question. The gas constant, r, is equal to 0.0821 when. 2311 l 2311 l 5) if i have 17.Ideal Gas Law Worksheet 144 Answer Key Greenus
Combined Gas Law Worksheet 1 Answer Key Organicked
Ideal gas law Packet 2 worksheet
Ideal Gas Law Worksheet 2 Answer Ideal Gas Law Worksheet PV = nRT Use
49 Ideal Gas Laws Worksheet Chessmuseum Template Library
Ideal Gas Law Worksheet Answers Questions Answer Zac Sheet
Worksheet K4 Chemistry Dalton's Law Law Worksheet
8 Best Images of Gas Laws Worksheet Answer Key Ideal Gas Law
Gas law packet answers Worksheet Template Tips And Reviews
8 Best Images of Chemistry Gas Laws Worksheet Ideal Gas Law Worksheet
Web Quiz & Worksheet Goals.
On This Worksheet You Will Practice With The Ideal Gas Law, The Combined Gas Law, As Well As The Relationships Between The Number Of Moles, The Mass, And The.
1) How Many Moles Of Gas Does It Take To Occupy 120 Liters At A Pressure Of 2.3.
Web Solutions To The Ideal Gas Law Practice Worksheet:
Related Post: