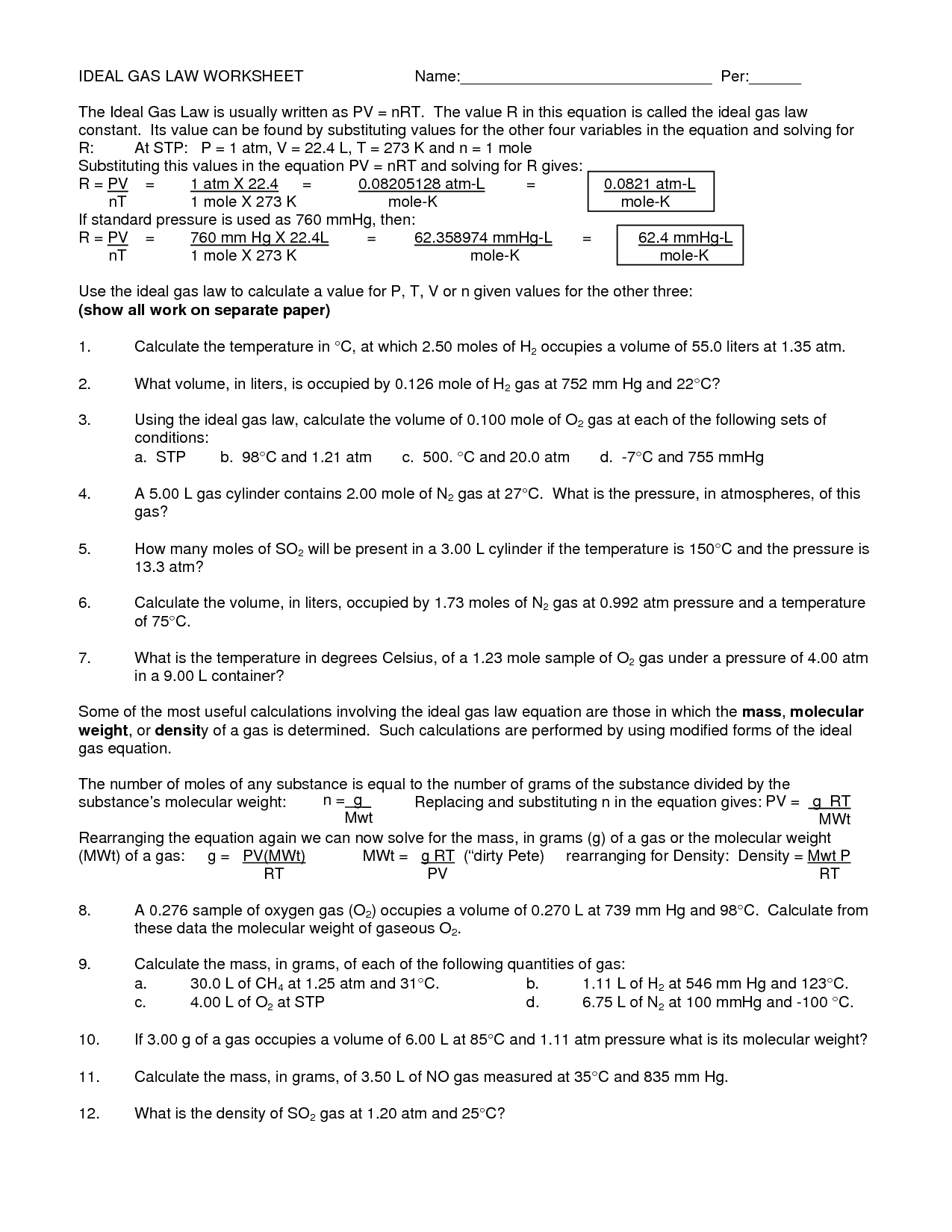
Ideal Gas Law Practice Worksheet
Ideal Gas Law Practice Worksheet - Web the ideal gas law (pv = nrt) worked example: Using the ideal gas law to calculate a change in. State what the 'n' represents in the ideal gas equation. Pv = nrt each quantity in the equation is usually expressed in the following units: How many moles of gas does it take to occupy 120 liters at a pressure of 2 atmospheres. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is. If the original pressure was 1.00 atm, what would the final pressure be? 1) how many moles of gas does it take to occupy 120 liters at a pressure of2.3. N = 0.00831 mol t = 25°c b) p = ? Web ideal gas law practice worksheet solve the following problems using the ideal gas law: State what the 'n' represents in the ideal gas equation. 1) how many moles of gas does it take to occupy 120.0 liters at a pressure of 2.3. Web ideal gas law directions: Web at low pressure (less than 1 atmosphere) and high temperature (greater. Web ideal gas law. Web ideal gas law name ___________ 1) given the following sets of values, calculate the unknown quantity. What is the volume at. To solve the following problems: Web these popular skills practice worksheets help your students master the ideal gas law. P = pressure, measured in atmospheres v = volume, measured in liters n = amount of gas, measured in moles If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of. Using the ideal gas law to calculate number of moles. What is the volume at. Web use. Web at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: Web ideal gas law directions: How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Web identify an ideal gas condition. Web ideal gas law practice worksheet solve the. P = pressure, measured in atmospheres v = volume, measured in liters n = amount of gas, measured in moles Web ideal gas law practice worksheet solve the following problems using the ideal gas law: Web ideal gas law name ___________ 1) given the following sets of values, calculate the unknown quantity. Assume that the lungs are at 1.00 atm. If the original pressure was 1.00 atm, what would the final pressure be? Web these popular skills practice worksheets help your students master the ideal gas law. Web use your knowledge of the ideal and combined gas laws to solve the following problems. P = pressure, measured in atmospheres v = volume, measured in liters n = amount of gas,. Web these popular skills practice worksheets help your students master the ideal gas law. What is the volume at. Solve the following problems using the ideal gas law: Web ideal gas law practice worksheet. P = pressure, measured in atmospheres v = volume, measured in liters n = amount of gas, measured in moles Describe what temperature a gas has to be in to use the ideal gas equation. How many moles of gas does it take to occupy 120 liters at a pressure of 2 atmospheres. If the original pressure was 1.00 atm, what would the final pressure be? Web ideal gas law practice worksheet. Web identify an ideal gas condition. Web ideal gas law worksheet pv = nrt. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. Solve the following problems using the ideal gas law: On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships between the number of moles,. Web ideal gas law practice worksheet solve the following problems using the ideal gas law: Web ideal gas law practice worksheet. To solve the following problems: 1) how many moles of gas does it take to occupy 120.0 liters at a pressure of 2.3. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v. 1) how many moles of gas does it take to occupy 120.0 liters at a pressure of 2.3. Web ideal gas law practice worksheet. To solve the following problems: With scaffolded questions, students will learn how to perform all of the calculations. Solve the following problems using the ideal gas law: 1) how many moles of gas does it take to occupy 120 liters at a pressure of2.3. Web ideal gas law directions: Web ideal gas law. Solutions to the ideal gas law practice worksheet: P = pressure, measured in atmospheres v = volume, measured in liters n = amount of gas, measured in moles Web at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: Pv = nrt each quantity in the equation is usually expressed in the following units: If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of. Web ideal gas law practice worksheet. Web ideal gas law name ___________ 1) given the following sets of values, calculate the unknown quantity. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. Using the ideal gas law to calculate number of moles. Web the ideal gas law (pv = nrt) worked example: Web ideal gas law practice worksheet solve the following problems using the ideal gas law: State what the 'n' represents in the ideal gas equation. Web ideal gas law practice worksheet solve the following problems using the ideal gas law: To solve the following problems: Web ideal gas law practice worksheet. Web ideal gas law practice worksheet solve the following problems using the ideal gas law: The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is. If the original pressure was 1.00 atm, what would the final pressure be? Web the ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas constant, and t is. 32) a gas occupies 1.00 l at standard temperature. Solutions to the ideal gas law practice worksheet: Web ideal gas law name ___________ 1) given the following sets of values, calculate the unknown quantity. N = 0.00831 mol t = 25°c b) p = ? If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of. Web ideal gas law. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. Web use your knowledge of the ideal and combined gas laws to solve the following problems. Web ideal gas law practice worksheet.15 Best Images of Ideal Gas Law Worksheet Ideal Gas Law Worksheet
Worksheet On Ideal Gas Equation What Ideal Gas Law Using the
SOLUTION Ideal gas law worksheet 2 answer Studypool
Avogadro's Law Worksheet Answers / Solved Experiment 14 Using The Ideal
Ideal Gas Law Worksheet
Worksheet On Ideal Gas Equation / IDEAL GAS EQUATION (11th Class) YouTube
Ideal Gas Law Practice Worksheet
Ideal Gas Law Worksheet Worksheet for 10th 12th Grade Lesson
15 Best Images of Ideal Gas Law Worksheet Ideal Gas Law Worksheet
Worksheet On Ideal Gas Equation / Ideal Gas Law Practice Worksheet
Web At Low Pressure (Less Than 1 Atmosphere) And High Temperature (Greater Than 0°C), Most Gases Obey The Ideal Gas Equation:
P = Pressure, Measured In Atmospheres V = Volume, Measured In Liters N = Amount Of Gas, Measured In Moles
Web These Popular Skills Practice Worksheets Help Your Students Master The Ideal Gas Law.
Using The Ideal Gas Law To Calculate Number Of Moles.
Related Post: