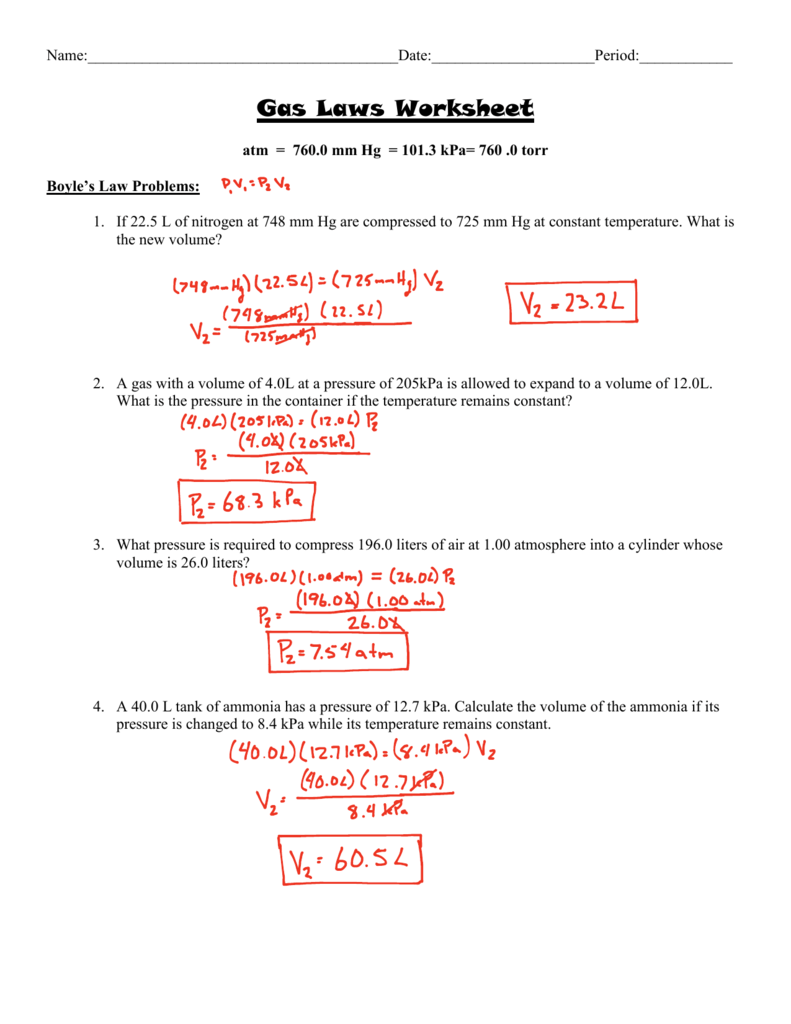
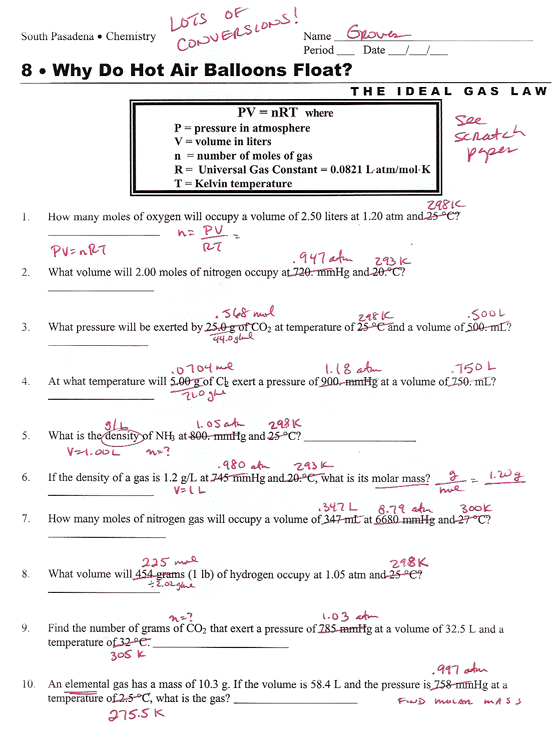
Ideal Gas Law Problems Worksheet Answers
Ideal Gas Law Problems Worksheet Answers - How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3 atmospheres and a temperature of 340 k?. Real gases behave like ideal gases except at very high temperatures. If i have an unknown quantity of gas at a pressure of 1.2 atm a volume of 31 liters, and a temperature of 87 0c, how many moles of gas do i have? Web solutions to the ideal gas law practice worksheet: Ideal gas law practice worksheet solve the following problems using the ideal gas law: Assume that the lungs are at 1.00 atm pressure and at a body temperature of 40 oc. Web the observed behavior of gases, embodied in the empirical gas laws, leads to a series of equations that can be summarized by a single equation of state, called the. Web 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc a gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is 740.0 mm of mercury. The ideal gas law investigates the relationship between pressure, volume, temperature, and moles of a gas. 1) how many moles of gas does it take to occupy 120 liters at a. Web 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc a gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c. P1v1 = p2v2 t1 t2 r = 8.31 l*kpa k*mol or. The gas constant, r, is equal to 0.0821 when. Ideal gas law questions and answers. If i have an unknown quantity of gas at a pressure of 1.2 atm a volume of 31 liters, and a temperature of 87 0c, how many moles of gas do i have? The. Web 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc a gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is 740.0 mm of mercury. Web the ideal gas law states that pv = nrt , where p is the pressure of a gas, v is the volume. The ideal gas law investigates the relationship between pressure, volume, temperature, and moles of a gas. Solutions to the ideal gas law practice worksheet: Assume that the lungs are at 1.00 atm pressure and at a body temperature of 40 oc. Web the observed behavior of gases, embodied in the empirical gas laws, leads to a series of equations that. Web solve the following problems using the ideal gas law: The ideal gas law name: The ideal gas law investigates the relationship between pressure, volume, temperature, and moles of a gas. Solve the following problems using the ideal gas law: Assume that the lungs are at 1.00 atm pressure and at a body temperature of 40 oc. If i have an unknown quantity of gas at a pressure of 1.2 atm a volume of 31 liters, and a temperature of 87 0c, how many moles of gas do i have? Web solutions to the ideal gas law practice worksheet: The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the. Real gases behave like ideal gases except at very high temperatures. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3 atmospheres and a temperature of 340 k?. Ideal gas law questions and answers. Web solutions to the ideal gas law practice worksheet: The gas constant, r, is equal to 0.0821 when. Ideal gas law questions and answers. 1) how many moles of gas does it take to occupy 120 liters at a. Solutions to the ideal gas law practice worksheet: Ideal gas law practice worksheet solve the following problems using the ideal gas law: Web 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc a gas balloon. Web the ideal gas law states that pv = nrt , where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas constant, and t is. Web the ideal gas law problems worksheet with answers is a great tool to help students. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3 atmospheres and a temperature of 340 k?. The ideal gas law name: How many moles of gas does it take to occupy 120 liters at a pressure of 2 atmospheres. The ideal gas law states that pv=nrt, where p is the pressure. Real gases behave like ideal gases except at very high temperatures. Ideal gas law questions and answers. The gas constant, r, is equal to 0.0821 when. Choose an answer and hit 'next'. Web up to 24% cash back the ideal gas law pv = nrt and combined gas laws rearrange the ideal gas law to solve for r and write it below: Web the ideal gas law problems worksheet with answers is a great tool to help students better understand the concept of the ideal gas law. The ideal gas law name: Solve each of the following problems. Web solve the following problems using the ideal gas law: Ideal gas law practice worksheet solve the following problems using the ideal gas law: 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3 atmospheres and a temperature of 340 k?. Web 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc a gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is 740.0 mm of mercury. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? 1) how many moles of gas does it take to occupy 120 liters at a. Indonesian kids want to find a way to. Assume that the lungs are at 1.00 atm pressure and at a body temperature of 40 oc. If i have an unknown quantity of gas at a pressure of 1.2 atm a volume of 31 liters, and a temperature of 87 0c, how many moles of gas do i have? Web write your answer on the line provided. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is. Web 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc a gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is 740.0 mm of mercury. Real gases behave like ideal gases except at very high temperatures. Assume that the lungs are at 1.00 atm pressure and at a body temperature of 40 oc. Web the observed behavior of gases, embodied in the empirical gas laws, leads to a series of equations that can be summarized by a single equation of state, called the. Ideal gas law practice worksheet solve the following problems using the ideal gas law: Choose an answer and hit 'next'. Solutions to the ideal gas law practice worksheet: Web ideal gas law practice worksheet. Web the ideal gas law states that pv = nrt , where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas constant, and t is. Web solve the following problems using the ideal gas law: Web solutions to the ideal gas law practice worksheet: The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of. The ideal gas law investigates the relationship between pressure, volume, temperature, and moles of a gas. Solve the following problems using the ideal gas law: The ideal gas law name:Worksheet On Ideal Gas Equation / Ideal Gas Law Practice Worksheet
Charles Law Worksheet Answers Pdf » Judithcahen Answer Key For Practice
Gas Laws Worksheet Answer Key
16 Pressure Problems Worksheet Answer Key /
Avogadro's Law Worksheet Answers / Solved Experiment 14 Using The Ideal
Ideal Gas Law Worksheet Answer Key —
Worksheet Ideal Gas Law Gas Density and Molar Mass With Answers II
13 Best Images of Pressure Problems Worksheet Answer Key
Ideal Gas Law Worksheet 144 Answer Key Greenus
SOLUTION Ideal gas law worksheet 2 answer Studypool
The Gas Constant, R, Is Equal To 0.0821 When.
Indonesian Kids Want To Find A Way To.
Web Up To 24% Cash Back The Ideal Gas Law Pv = Nrt And Combined Gas Laws Rearrange The Ideal Gas Law To Solve For R And Write It Below:
Ideal Gas Law Questions And Answers.
Related Post: