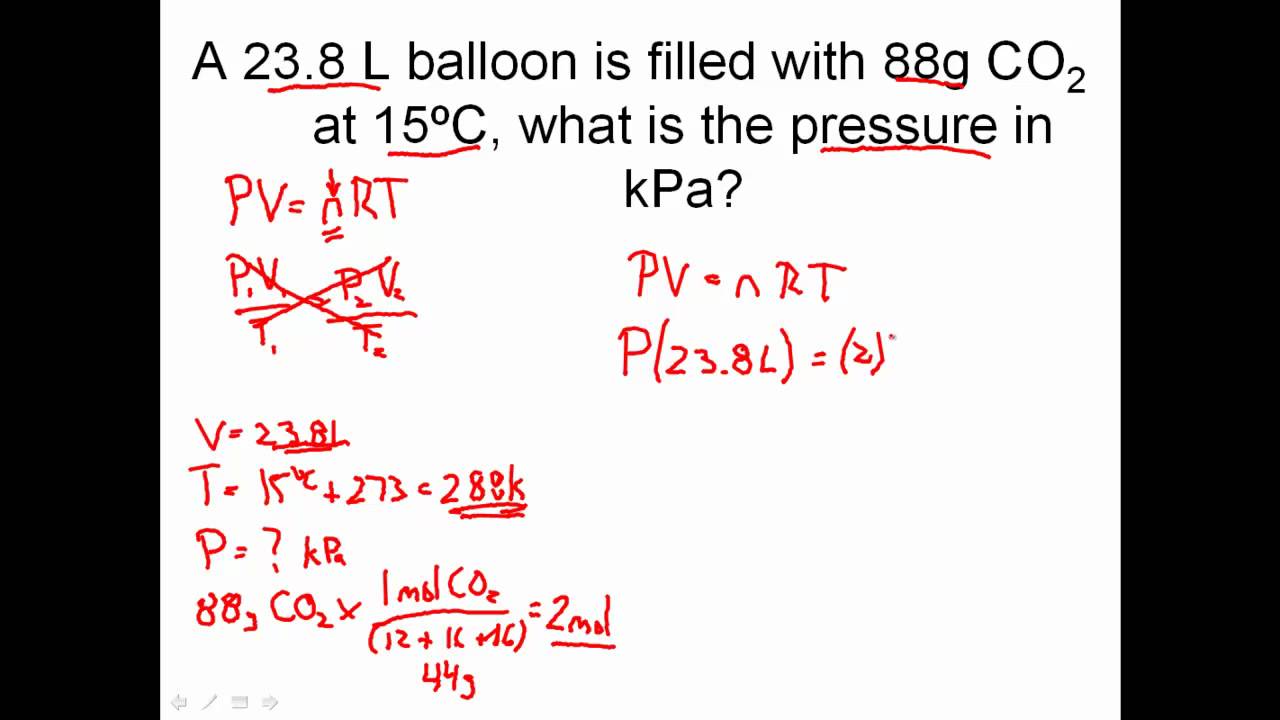
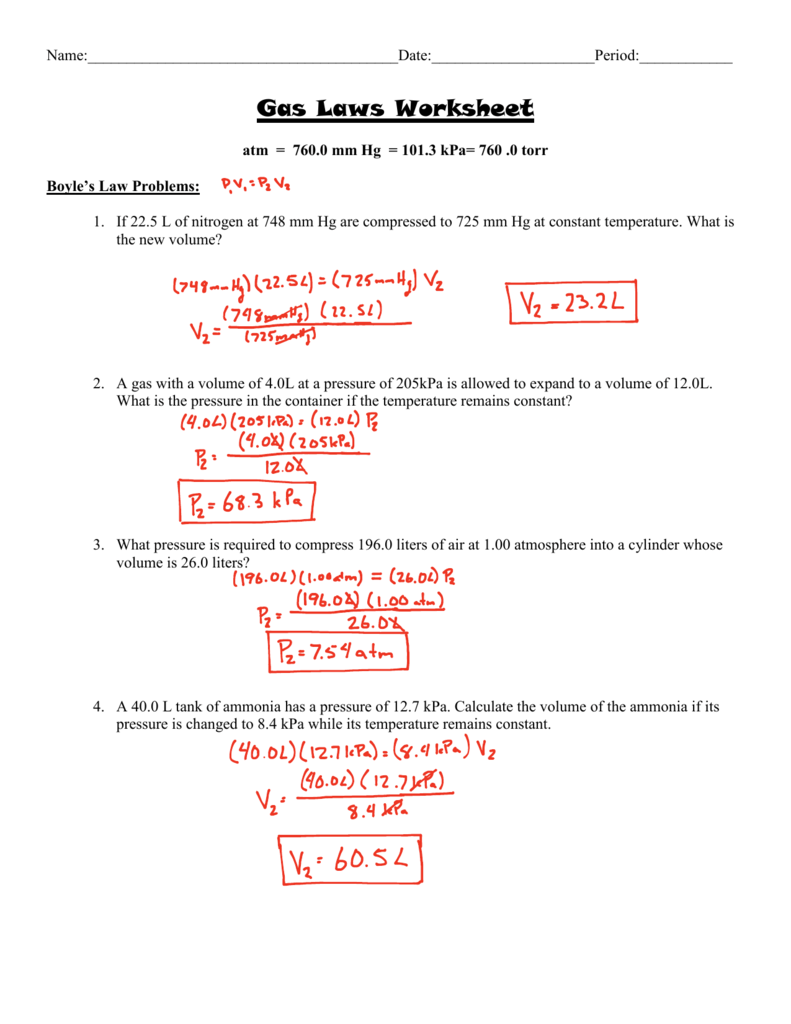
Ideal Gas Law Problems Worksheet
Ideal Gas Law Problems Worksheet - Describe what temperature a gas has to be in to use the ideal gas equation. The gas constant, r, is equal to 0.0821. If 15.0 liters of neon at 25.0 °c is allowed to expand to 45.0 liters, what must the new temperature be to maintain constant pressure? Web this labeling worksheet allows students to practice the ideal gas law and combined gas law formulas while reinforcing the identification of variables and units in practice and. Solve the following problems using the ideal gas law: Oxygen gas is collected at a pressure of 123 kpa in a container which has a volume of 10.0 l. Web identify an ideal gas condition. This worksheet has 10 calculation problems involving the ideal gas law. The ideal gas law (pv = nrt) worked example: State what the 'n' represents in the ideal gas equation. This worksheet has 10 calculation problems involving the ideal gas law. Web 1) given the following sets of values, calculate the unknown quantity. Web in ideal gas law problems, one variable is solved at a time using the equation {eq}pv=nrt {/eq} example 1: N = 0.00831 mol t = 25°c b) p = ? Web solutions gases 1 (worksheet) “.it. Web gas laws packet #2. The gas constant, r, is equal to 0.0821. Web ideal gas law practice worksheet. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. N = 0.00831 mol t = 25°c b) p = ? Web gas laws packet #2. For any sample of gas under ideal conditions, the relationship between the amount of gas in moles (n) and its temperature, pressure, and volume is. Using the ideal gas law to calculate number of moles. Solve the following problems using the ideal gas law: Web then p = n = v = t = r. What temperature must be maintained on 0.500 moles of this gas in order to maintain this pressure? Using the ideal gas law to calculate. There are no conversions here. Describe what temperature a gas has to be in to use the ideal gas equation. 1) how many moles of gas does it take to occupy 120 liters at a pressure. On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships between the number of moles, the. Web ideal gas law practice worksheet. Web ideal gas law practice worksheet. The gas constant, r, is equal to 0.0821. 1) how many moles of gas does it take to occupy 120 liters at. State what the 'n' represents in the ideal gas equation. For any sample of gas under ideal conditions, the relationship between the amount of gas in moles (n) and its temperature, pressure, and volume is. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. Web identify an ideal gas condition. The. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. State what the 'n' represents in the ideal gas equation. Using the ideal gas law to calculate. Web ideal gas law practice worksheet. Web ideal gas law. Web identify an ideal gas condition. Web what is the volume at 132.0 °c? The ideal gas law (pv = nrt) worked example: Solve the following problems using the ideal gas law: Web solutions gases 1 (worksheet) “.it was as though scales fell from my eyes, doubt vanished, and was replaced by a feeling of peaceful calm.” lothar meyer, upon. Web ideal gas law. Web then p = n = v = t = r = what pressure is required to contain 0.023 moles of nitrogen gas in a 4.2 l container at a temperature of 20.(c? If 15.0 liters of neon at 25.0 °c is allowed to expand to 45.0 liters, what must the new temperature be to maintain. Oxygen gas is collected at a pressure of 123 kpa in a container which has a volume of 10.0 l. Web solutions gases 1 (worksheet) “.it was as though scales fell from my eyes, doubt vanished, and was replaced by a feeling of peaceful calm.” lothar meyer, upon hearing avogadro’s hypothesis it took chemists a long time to understand the. State what the 'n' represents in the ideal gas equation. Web gas laws packet #2. Web 1) given the following sets of values, calculate the unknown quantity. The ideal gas law (pv = nrt) worked example: Web solutions gases 1 (worksheet) “.it was as though scales fell from my eyes, doubt vanished, and was replaced by a feeling of peaceful calm.” lothar meyer, upon hearing avogadro’s hypothesis it took chemists a long time to understand the nature of gases. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. What temperature must be maintained on 0.500 moles of this gas in order to maintain this pressure? Web ideal gas law. Web in ideal gas law problems, one variable is solved at a time using the equation {eq}pv=nrt {/eq} example 1: If 15.0 liters of neon at 25.0 °c is allowed to expand to 45.0 liters, what must the new temperature be to maintain constant pressure? Students just need to solve the ideal gas equation for the correct. Web this labeling worksheet allows students to practice the ideal gas law and combined gas law formulas while reinforcing the identification of variables and units in practice and. V= 0.602 l n = 0.00801 mol t = 311 k 2) at what. Oxygen gas is collected at a pressure of 123 kpa in a container which has a volume of 10.0 l. Using the ideal gas law to calculate number of moles. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. Web then p = n = v = t = r = what pressure is required to contain 0.023 moles of nitrogen gas in a 4.2 l container at a temperature of 20.(c? The gas constant, r, is equal to 0.0821. Web ideal gas law practice worksheet. There are no conversions here. Describe what temperature a gas has to be in to use the ideal gas equation. V= 0.602 l n = 0.00801 mol t = 311 k 2) at what. Web ideal gas law. Web in ideal gas law problems, one variable is solved at a time using the equation {eq}pv=nrt {/eq} example 1: Ideal gas law worksheet pv = nrt. Web solutions gases 1 (worksheet) “.it was as though scales fell from my eyes, doubt vanished, and was replaced by a feeling of peaceful calm.” lothar meyer, upon hearing avogadro’s hypothesis it took chemists a long time to understand the nature of gases. Students just need to solve the ideal gas equation for the correct. Oxygen gas is collected at a pressure of 123 kpa in a container which has a volume of 10.0 l. Real gases behave like ideal gases except at very high temperatures. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. Web then p = n = v = t = r = what pressure is required to contain 0.023 moles of nitrogen gas in a 4.2 l container at a temperature of 20.(c? On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships between the number of moles, the. How many moles of gas does it take to occupy 120 liters at a pressure of 2 atmospheres. What temperature must be maintained on 0.500 moles of this gas in order to maintain this pressure? Web 1) given the following sets of values, calculate the unknown quantity. If 15.0 liters of neon at 25.0 °c is allowed to expand to 45.0 liters, what must the new temperature be to maintain constant pressure?IDEAL GAS LAW PRACTICE PROBLEMS How to Solve Ideal Gas Law Problems
Ideal Gas Law Problems Worksheet Worksheet
Worksheet Gas Laws Chemistry At Central High School Worksheet
Ideal Gas Law Practice Worksheet
Avogadro's Law Worksheet Answers / Solved Experiment 14 Using The Ideal
SOLUTION Ideal gas law worksheet 2 answer Studypool
15 Best Images of Ideal Gas Law Worksheet Ideal Gas Law Worksheet
8 Best Images of Gas Law Problems Worksheet Combined Gas Law Problems
Gas Laws Worksheet 1
Ideal Gas Law Worksheet Worksheet for 10th 12th Grade Lesson
Web Ideal Gas Law Practice Worksheet Solve The Following Problems Using The Ideal Gas Law:
N = 0.00831 Mol T = 25°C B) P = ?
A) P = 1.01 Atm V = ?
The Ideal Gas Law (Pv = Nrt) Worked Example:
Related Post: