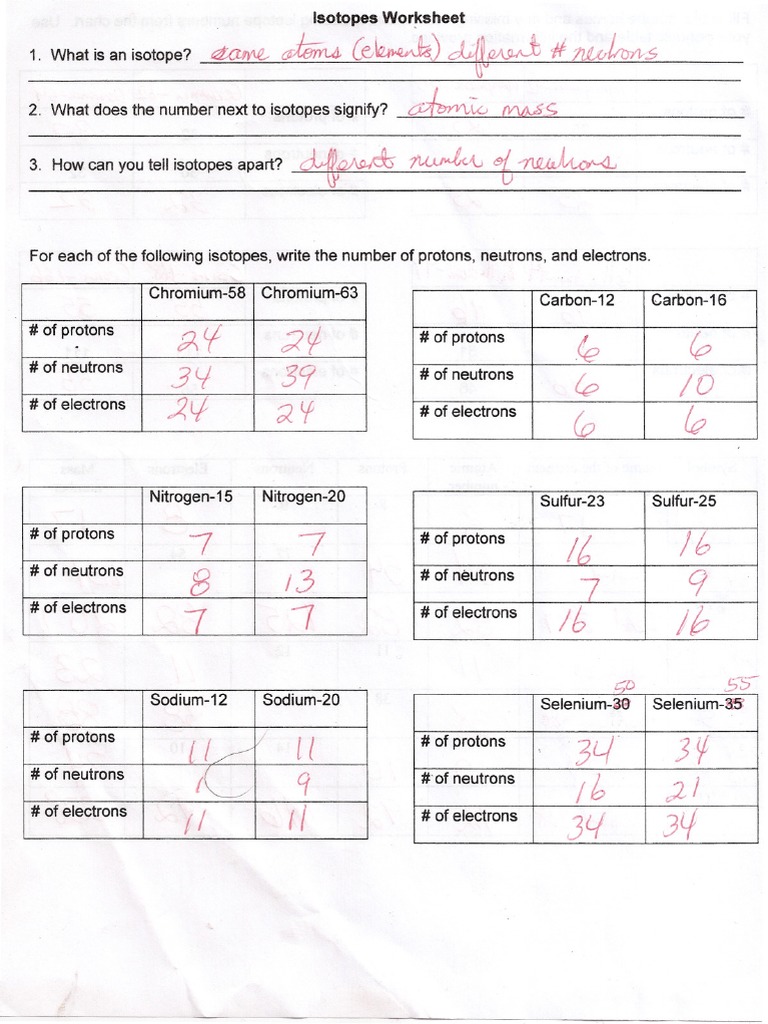
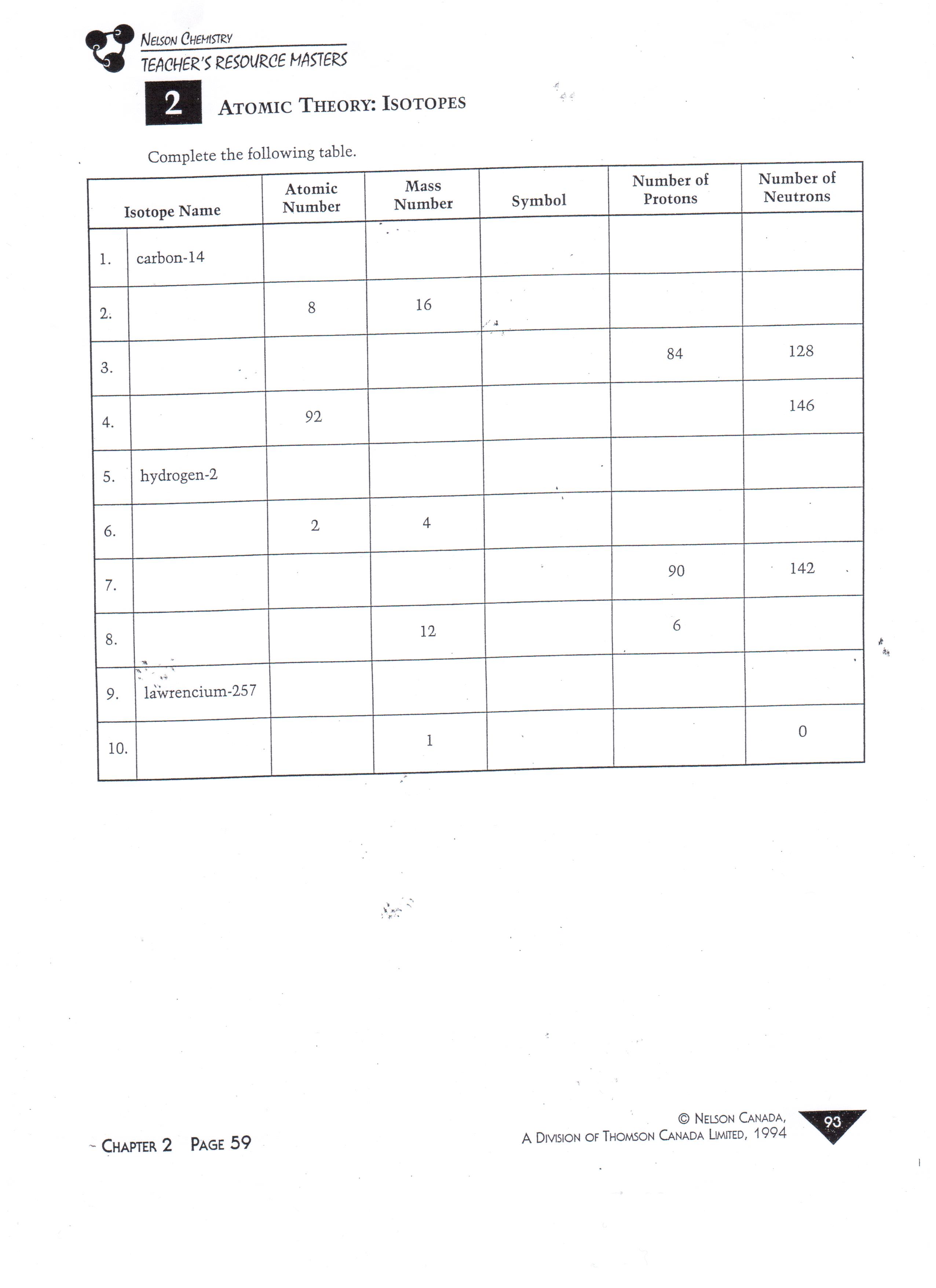
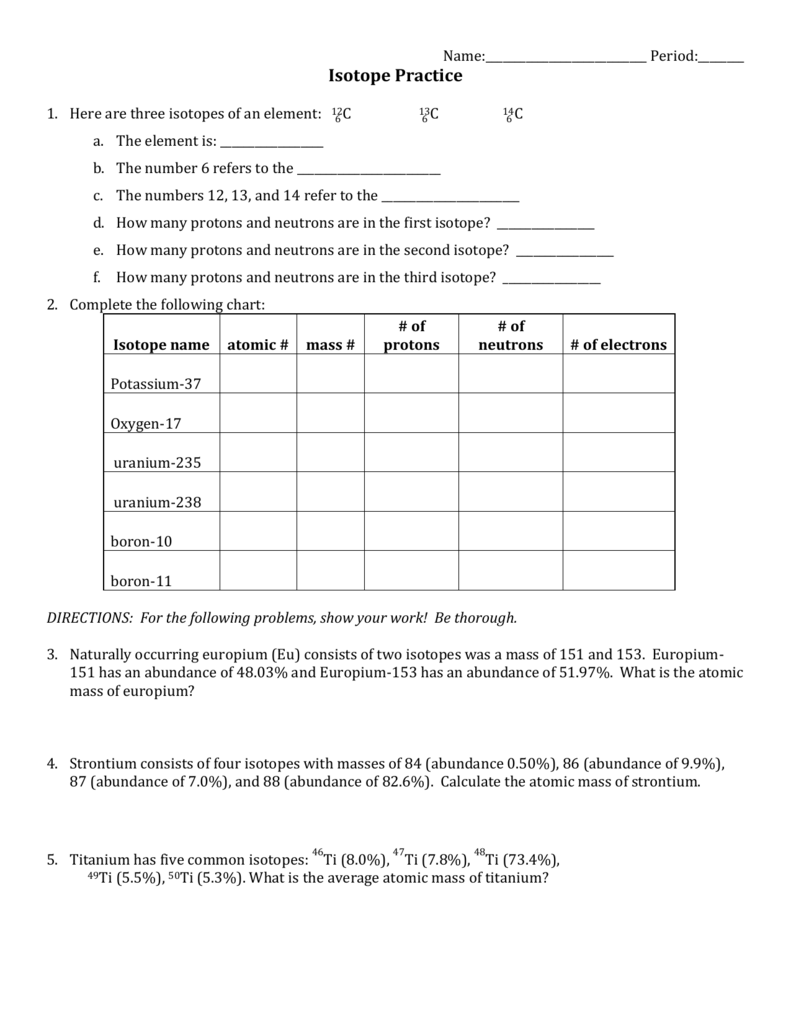
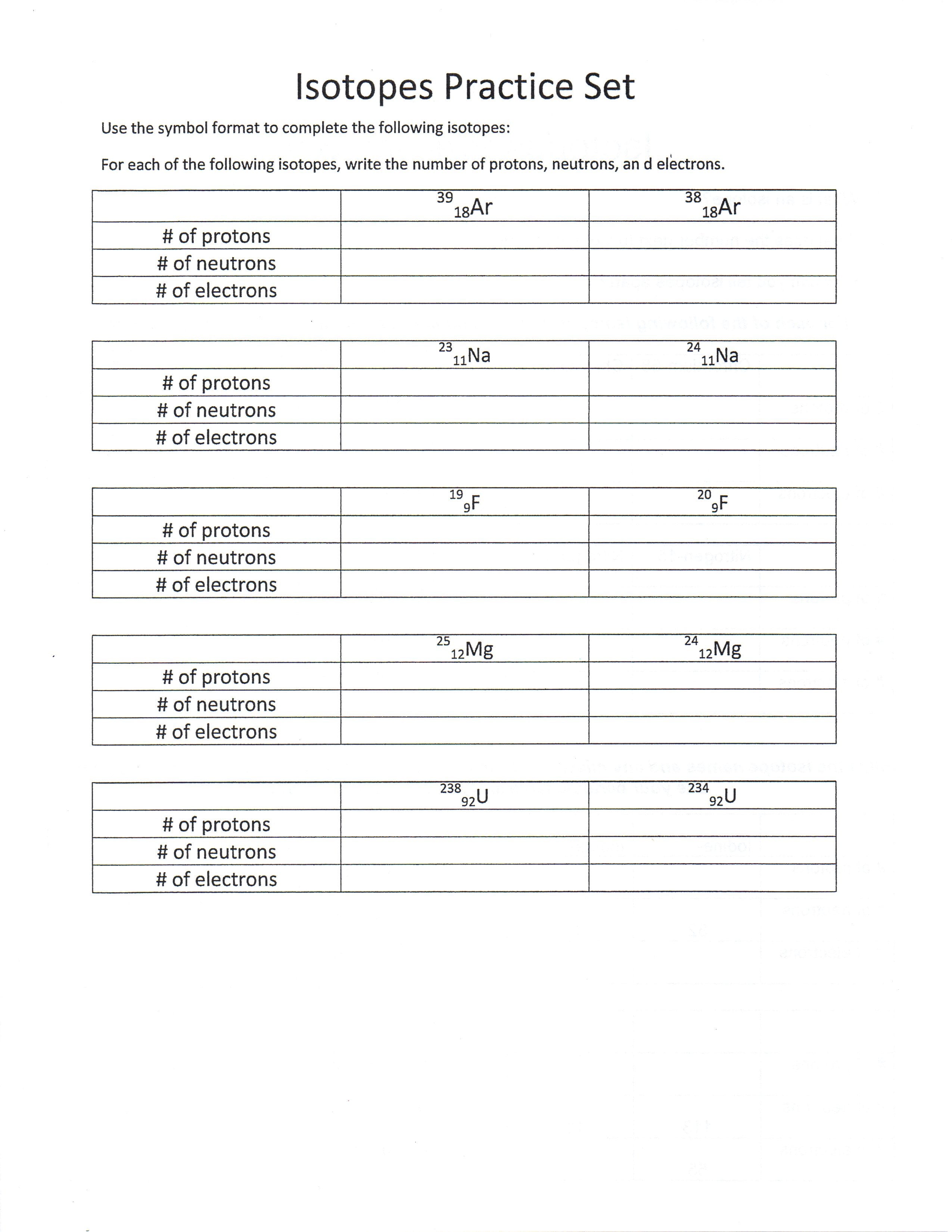
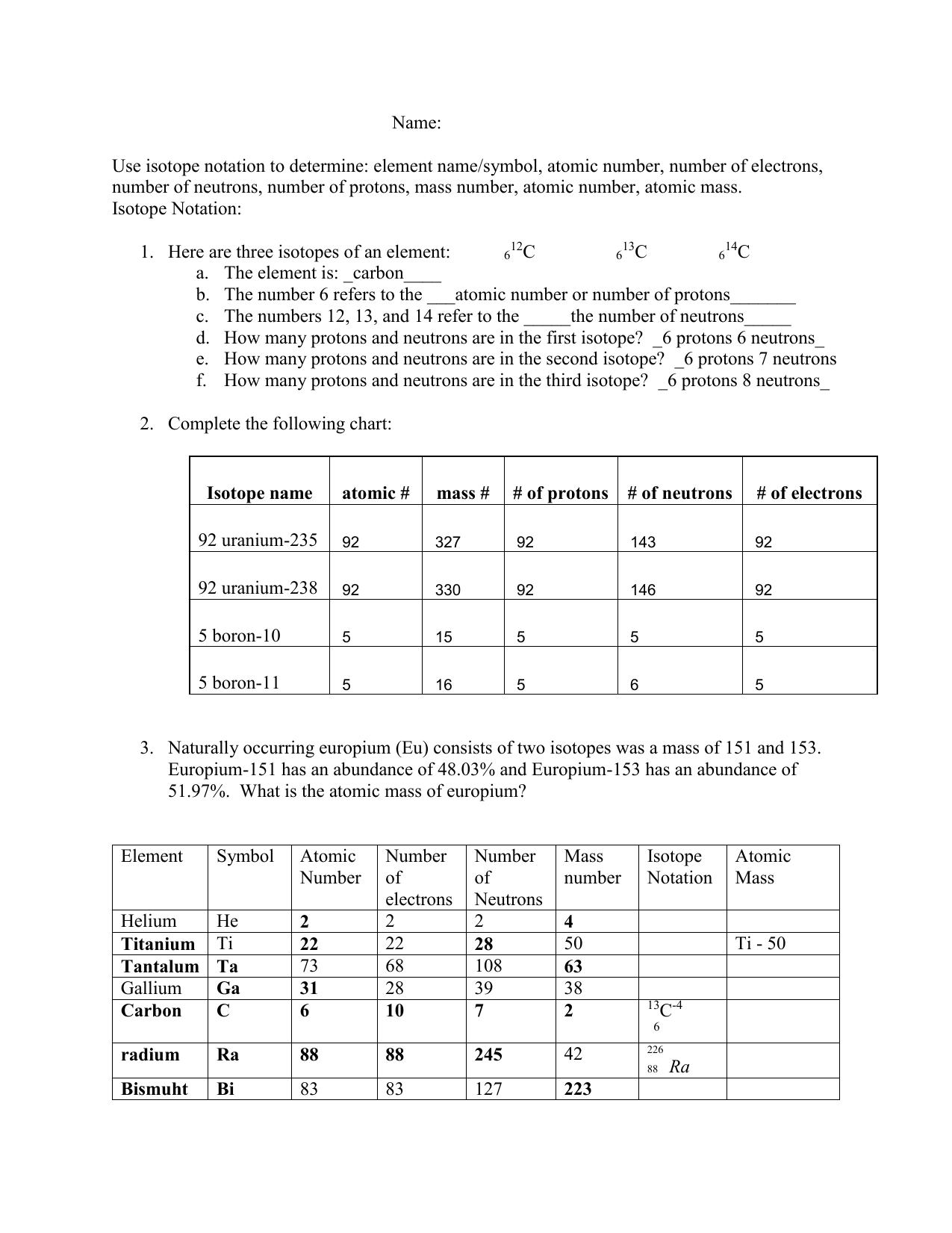
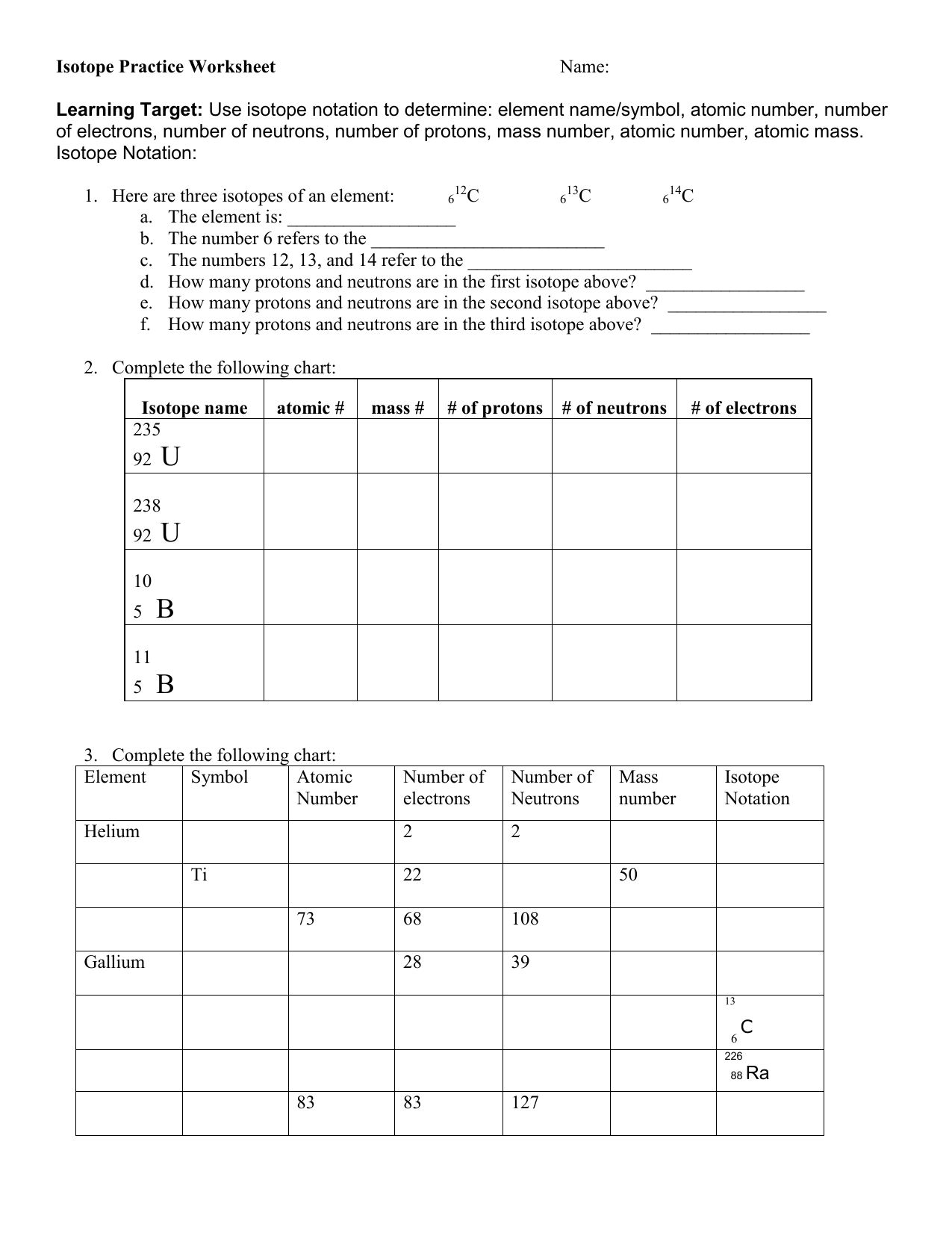
Isotope Practice Worksheet
Isotope Practice Worksheet - Which subatomic particles stay the same in isotopes of the same element? Isotopes are versions of the same element. Here are three isotopes of an element: Web isotopes worksheets and online activities. Web atoms and isotopes worksheet fill in the table with the correct information 1. Use the periodic table to identify and count subatomic particles within the atom. Many exist permanently, while others may only exist briefly before decomposing into new elements. Web isotope practice worksheet name: 12c 6 13c 14c 6 6 a. A chemical element’s isotope is a distinct form with a different number of neutrons in its nucleus. A chemical element’s isotope is a distinct form with a different number of neutrons in its nucleus. The number 6 refers to the atomic number c. How many protons and neutrons are in the first isotope? What subatomic particle is different in isotopes of the same element? Web isotopes worksheet part i. 50.54% of the naturally occurring isotopes of bromine have an atomic mass of 78.92 u while 49.46% of the naturally occurring isotopes of bromine have an atomic mass of 80.92 u. For the questions below determine if the particle that is described in an atom or an ion. 6 12c 6 13c 6 14c a. Web isotope practice worksheet 1.. Web for each of the following isotopes, write the # of protons, neutrons, and electrons. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons. I can use the atomic number of an element to identify protons and electrons for any element. Web isotope practice worksheet name: How many protons. Web isotope practice worksheet name: Web isotope and ions practice worksheet name: Web isotope practice worksheet 1. What does the number next to isotopes signify? Some of the worksheets for this concept are isotopesions work element element atomic mass, , isotope practice work, isotopes, atom ion isotopes work, scanned from a xerox multifunction er, isotopes, isotopes work with answers. 12 c 6 13 c 6 14 c 6 a. Describe the general arrangement of subatomic particles in the atom electrons surround the nucleus; Use the periodic table to identify and count subatomic particles within the atom. Web atoms and isotopes worksheet fill in the table with the correct information 1. Web isotope worksheet write the most common isotope in. Web isotopes worksheets and online activities. Use your periodic table and the information provided. Web isotope practice worksheet 1. Answer the questions based on the above reading. Here are three isotopes of an element: How many protons and neutrons are in the first. Web in this practice worksheet students will demonstrate understanding of isotopes and ions. What does the number next to isotopes signify? Describe the general arrangement of subatomic particles in the atom electrons surround the nucleus; For the questions below determine if the particle that is described in an atom or an. The number 6 refers to the _________________________c. 12 c 6 13 c 6 14 c 6 a. Many exist permanently, while others may only exist briefly before decomposing into new elements. How many protons and neutrons are in the second isotope? For the questions below determine if the particle that is described in an atom or an ion. How many protons and neutrons are in the first isotope? The majority of elements have several isotopes. Web in this practice worksheet students will demonstrate understanding of isotopes and ions. The number 6 refers to the _________________________c. Here are three isotopes of an element: Web free collection of isotope practice worksheets for students. Web isotope practice worksheet name: Many exist permanently, while others may only exist briefly before decomposing into new elements. Web isotope practice worksheet 1. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons. How many protons and neutrons are in the first isotope? Here are three isotopes of an element: How do atoms become cations and anions? Web this is a worksheet of extra practice problems for students who struggled with the ions and ion notation worksheet, and/or the isotopes and isotope notation worksheet. Some of the worksheets for this concept are isotopesions work element element atomic mass, , isotope practice work, isotopes, atom ion isotopes work, scanned from a xerox multifunction er, isotopes, isotopes work with answers. For the questions below determine if the particle that is described in an atom or an ion. How do atoms become isotopes? What would happen if the number of protons were to change in an atom? Web isotope practice worksheet 1. Use the periodic table to identify and count subatomic particles within the atom. How many protons and neutrons are in the first isotope? Add to my workbooks (38) download file pdf embed in my website or blog add to google classroom 50.54% of the naturally occurring isotopes of bromine have an atomic mass of 78.92 u while 49.46% of the naturally occurring isotopes of bromine have an atomic mass of 80.92 u. Web isotopes worksheets and online activities. The number 6 refers to the atomic number c. Web isotope practice worksheet 1. 6 12c 6 13c 6 14c a. How many protons and neutrons are in the second isotope? A student looked up the naturally occurring isotopes of bromine and found the following information: Web isotopes worksheet part i. Web this is a worksheet of extra practice problems for students who struggled with the ions and ion notation worksheet, and/or the isotopes and isotope notation worksheet. Web isotopes worksheets and online activities. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons. A chemical element’s isotope is a distinct form with a different number of neutrons in its nucleus. Answer the questions based on the above reading. I can use the atomic number of an element to identify protons and electrons for any element. Students are given a simple table that gives limited information. 50.54% of the naturally occurring isotopes of bromine have an atomic mass of 78.92 u while 49.46% of the naturally occurring isotopes of bromine have an atomic mass of 80.92 u. Isotopes are versions of the same element. 6 12c 6 13c 6 14c a. The number 6 refers to the _________________________c. 12 c 6 13 c 6 14 c 6 a. The most common isotope can be found by rounding the atomic mass found on the periodic table of elements to the nearest whole number. Describe the general arrangement of subatomic particles in the atom electrons surround the nucleus; Web in this practice worksheet students will demonstrate understanding of isotopes and ions. Web for each of the following isotopes, write the # of protons, neutrons, and electrons.5 Isotope Practice Worksheet
Isotopes Worksheet Answers
30 isotope Practice Worksheet Answers Education Template
Most Common Isotope Worksheet 1
Isotope Practice Worksheet
Isotopes Worksheet Answer Key
Isotope Practice Worksheet Answers
Isotope Notation Chem Worksheet 4 2 —
Isotope Practice Worksheet Answer Key
Isotope Practice Worksheet
What Does The Number Next To Isotopes Signify?
The Numbers 12, 13, And 14 Refer To The Mass Number D.
Calculate The Average Atomic Mass Of Bromine, Showing All Work:
How Many Protons And Neutrons Are In The First Isotope?
Related Post: