Isotopes Worksheet With Answers
Isotopes Worksheet With Answers - Chlorine consists of two isotopes with masses of 35 (abundance 75%) and 37 (abundance of 25%). An isotope is an atom of the same element that have the same number of protons and electrons but a different number of neutrons. Web see the answer below. Free interactive exercises to practice online or download as pdf to print. Web the answer has to do with the fact that different isotopes have different relative abundances, which means that some isotopes are more naturally abundant on earth. (a) given that the relative abundances of cl 35.00 and cl 37.00 are 77.50% and 22.50% respectively, calculate the average relative atomic mass of chlorine atoms to four significant figures. Calculate the relative atomic mass of strontium. What contribution did these scientists make to atomic. Fill in the isotope names and any missing information, including isotope numbers from the chart. Web 37.00 (the m/z peaks on a mass spectrum identify the different isotopes of an element that are present in the sample). Fill in the isotope names and any missing information, including isotope numbers from the chart. This worksheet provides 18 examples for students to determine the protons, electrons, neutrons, atomic #, mass #, hyphenated name, and isotope symbols. Add to my workbooks (38) download file pdf embed in. As a result in the. Web 88ra 83 83 127 isotope practice worksheet. (a) given that the relative abundances of cl 35.00 and cl 37.00 are 77.50% and 22.50% respectively, calculate the average relative atomic mass of chlorine atoms to four significant figures. An atom consists of a nucleus and electrons surrounding the nucleus. An isotope is an atom of the same element that have the same number of protons and electrons but. Web isotopes work sheet. The components of an atom. Chlorine consists of two isotopes with masses of 35 (abundance 75%) and 37 (abundance of 25%). Fill in the table with the correct information. Web 37.00 (the m/z peaks on a mass spectrum identify the different isotopes of an element that are present in the sample). Protons/neutrons (marbles, counters, etc.) 2 dice (symbols and numbers) atomic bowl safety: An isotope is an atom of the same element that have the same number of protons and electrons but a different number of neutrons. Web the answer has to do with the fact that different isotopes have different relative abundances, which means that some isotopes are more naturally. An isotope is an atom of the same element that have the same number of protons and electrons but a different number of neutrons. The components of an atom. (a) given that the relative abundances of cl 35.00 and cl 37.00 are 77.50% and 22.50% respectively, calculate the average relative atomic mass of chlorine atoms to four significant figures. There. There are no safety concerns associated with this activity. Web answer the following questions: Web 37.00 (the m/z peaks on a mass spectrum identify the different isotopes of an element that are present in the sample). Fill in the table with the correct information. Describe the general arrangement of subatomic particles in the atom. Web the answer has to do with the fact that different isotopes have different relative abundances, which means that some isotopes are more naturally abundant on earth. Use the periodic table to identify and count subatomic particles within the atom. Web see the answer below. Free interactive exercises to practice online or download as pdf to print. There are no. Exercise add to my workbooks (16) download file pdf. Chlorine consists of two isotopes with masses of 35 (abundance 75%) and 37 (abundance of 25%). Fill in the isotope names and any missing information, including isotope numbers from the chart. What contribution did these scientists make to atomic. Web the answer has to do with the fact that different isotopes. An isotope is an atom of the same element that have the same number of protons and electrons but a different number of neutrons. How can you tell if an atom has a positive. Web see the answer below. What contribution did these scientists make to atomic. How can you tell if an atom has a negative charge? (a) given that the relative abundances of cl 35.00 and cl 37.00 are 77.50% and 22.50% respectively, calculate the average relative atomic mass of chlorine atoms to four significant figures. An isotope is an atom of the same element that have the same number of protons and electrons but a different number of neutrons. An atom consists of a nucleus. Describe the general arrangement of subatomic particles in the atom. Web see the answer below. I can use the atomic number of an. Web for each of the following isotopes, write the # of protons, neutrons, and electrons. How can you tell if an atom has a positive. Web isotopes worksheets and online activities. Web answer the following questions: The components of an atom. Use the periodic table to identify and count subatomic particles within the atom. Chlorine consists of two isotopes with masses of 35 (abundance 75%) and 37 (abundance of 25%). This worksheet provides 18 examples for students to determine the protons, electrons, neutrons, atomic #, mass #, hyphenated name, and isotope symbols. Web 37.00 (the m/z peaks on a mass spectrum identify the different isotopes of an element that are present in the sample). Add to my workbooks (38) download file pdf embed in. Web isotopes work sheet. As a result in the. Fill in the isotope names and any missing information, including isotope numbers from the chart. Web 88ra 83 83 127 isotope practice worksheet name: An isotope is an atom of the same element that have the same number of protons and electrons but a different number of neutrons. What type of ion is this? Protons/neutrons (marbles, counters, etc.) 2 dice (symbols and numbers) atomic bowl safety: Web see the answer below. (a) given that the relative abundances of cl 35.00 and cl 37.00 are 77.50% and 22.50% respectively, calculate the average relative atomic mass of chlorine atoms to four significant figures. There are no safety concerns associated with this activity. What type of ion is this? Fill in the isotope names and any missing information, including isotope numbers from the chart. Calculate the relative atomic mass of strontium. An isotope is an atom of the same element that have the same number of protons and electrons but a different number of neutrons. Web isotopes worksheets and online activities. Web 88ra 83 83 127 isotope practice worksheet name: Chlorine consists of two isotopes with masses of 35 (abundance 75%) and 37 (abundance of 25%). Web the answer has to do with the fact that different isotopes have different relative abundances, which means that some isotopes are more naturally abundant on earth. As a result in the. Naturally occurring boron (b) consists of two isotopes with a mass of 10 and 11. What contribution did these scientists make to atomic. Use the periodic table to identify and count subatomic particles within the atom. An atom consists of a nucleus and electrons surrounding the nucleus.Isotopes_Worksheet_Answers (1).pdf
Isotope Practice Worksheet Answer Key
Isotopes Worksheet Answer Key
Isotope Practice Worksheet
30 isotope Practice Worksheet Answers Education Template
30 isotope Practice Worksheet Answers Education Template
Isotopes Worksheet Answer Key
Isotopes Worksheet Answers Practices worksheets, Word problem
Isotopes Worksheet Answer Key
Isotopes And Ions Worksheet Answers
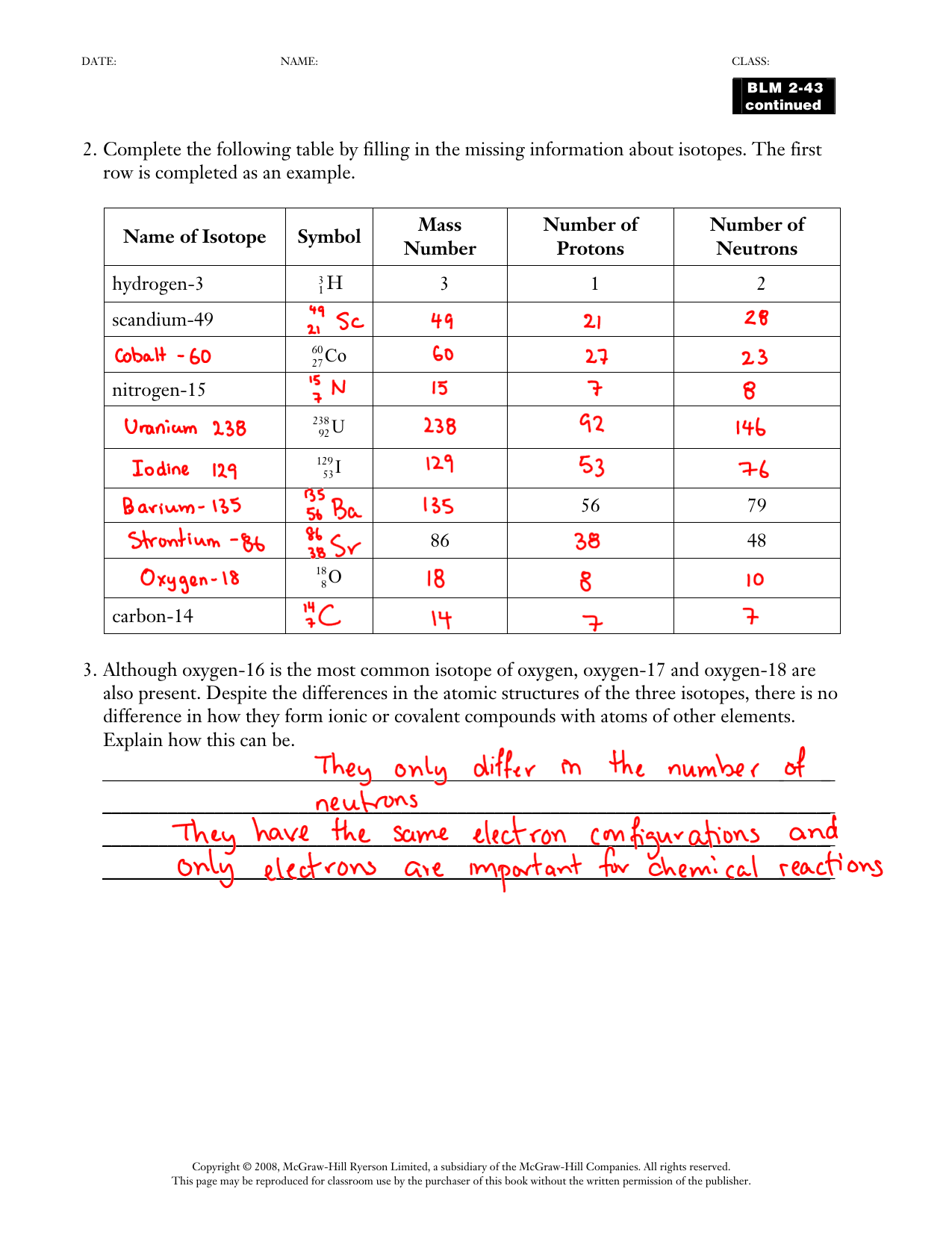
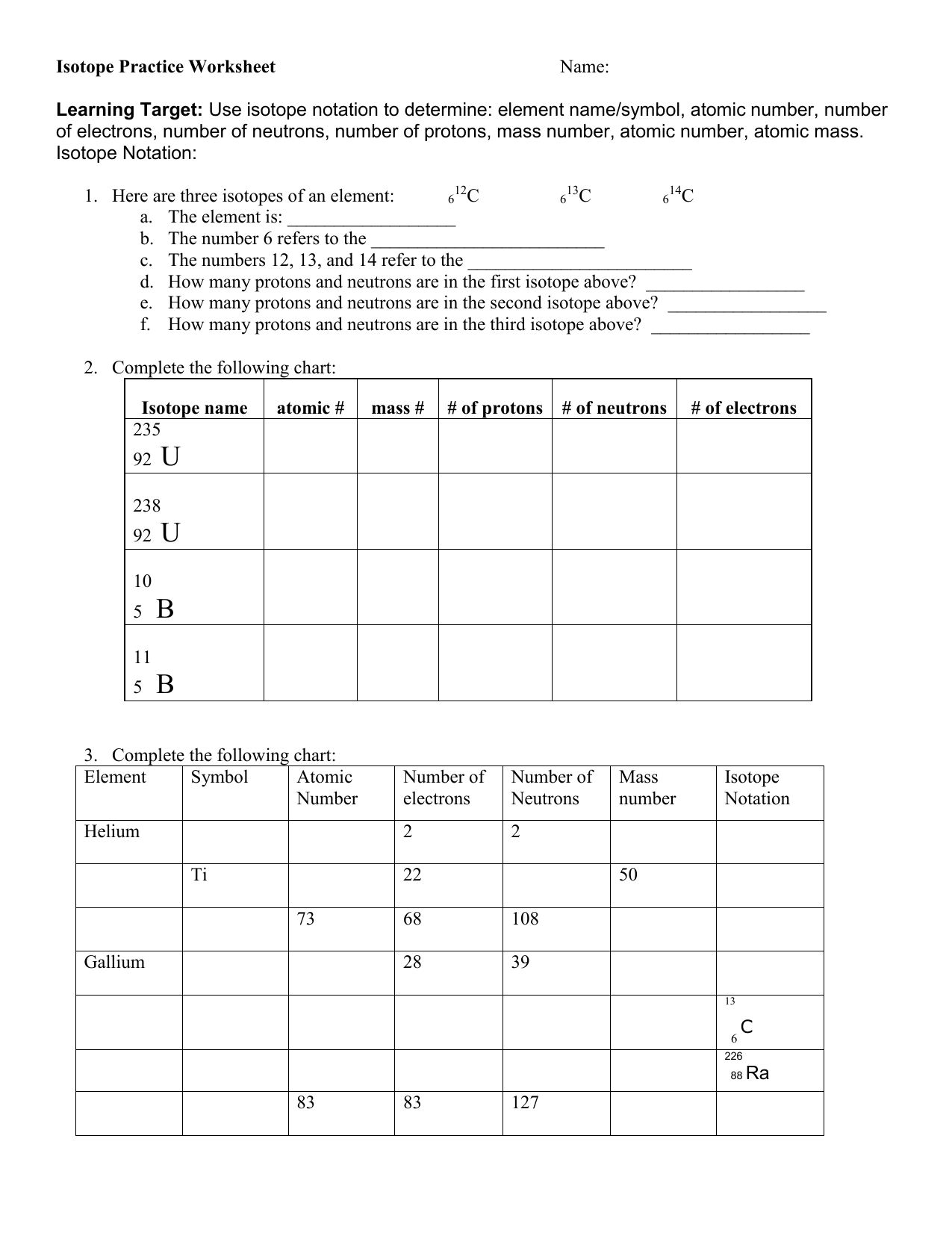
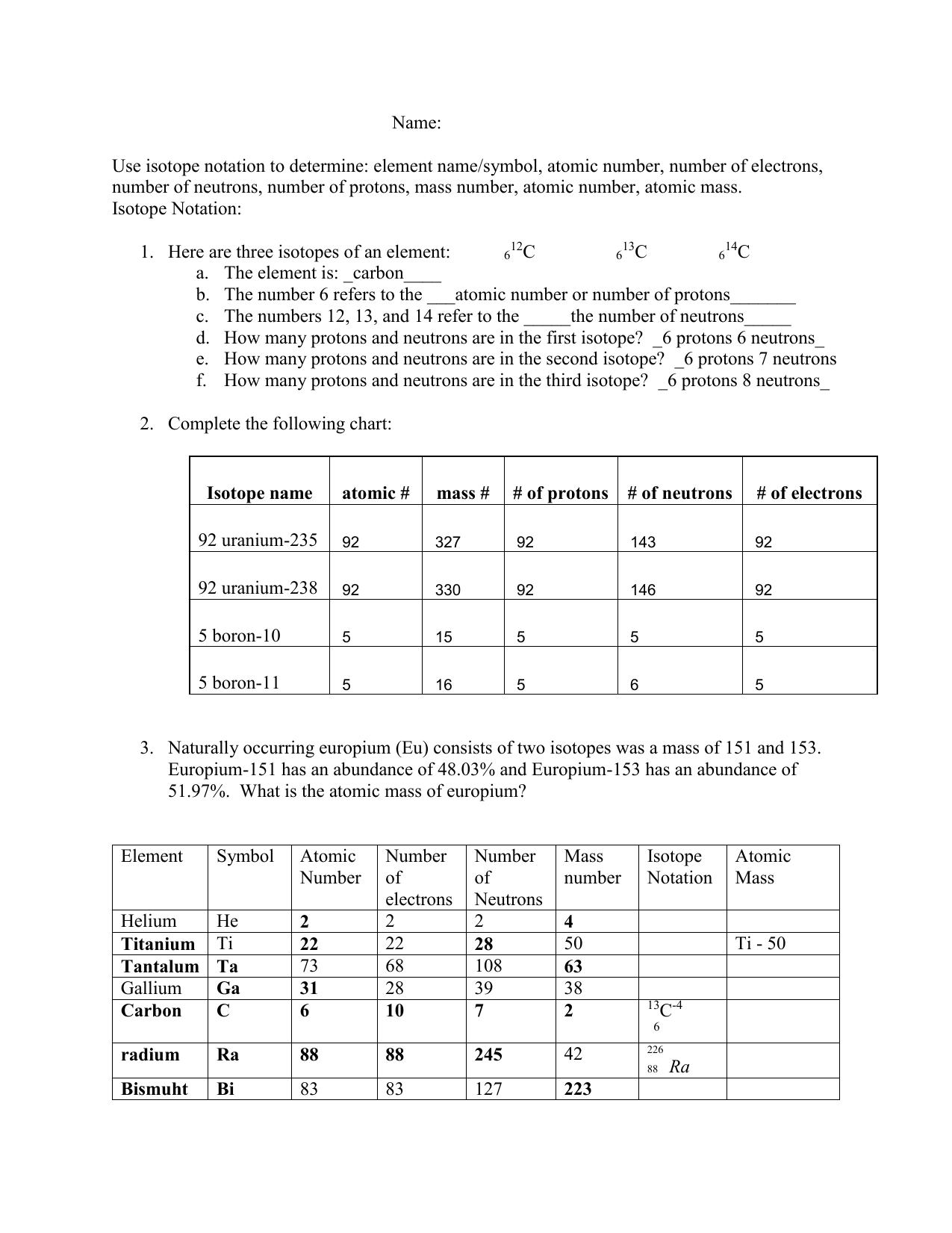
Fill In The Table With The Correct Information.
Web 37.00 (The M/Z Peaks On A Mass Spectrum Identify The Different Isotopes Of An Element That Are Present In The Sample).
Science Content For The Teacher:
Protons/Neutrons (Marbles, Counters, Etc.) 2 Dice (Symbols And Numbers) Atomic Bowl Safety:
Related Post: