Lewis Structures And Vsepr Worksheet
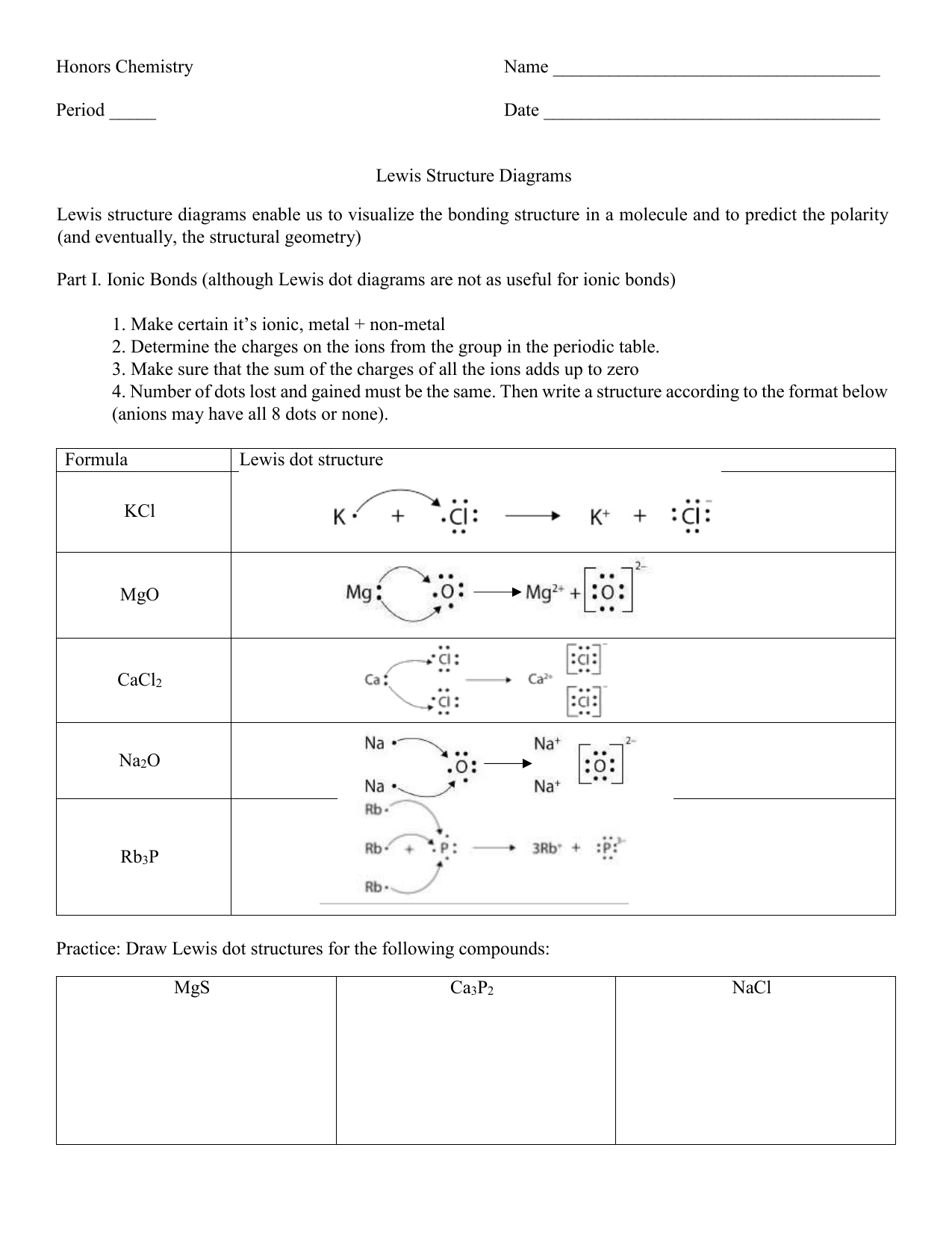
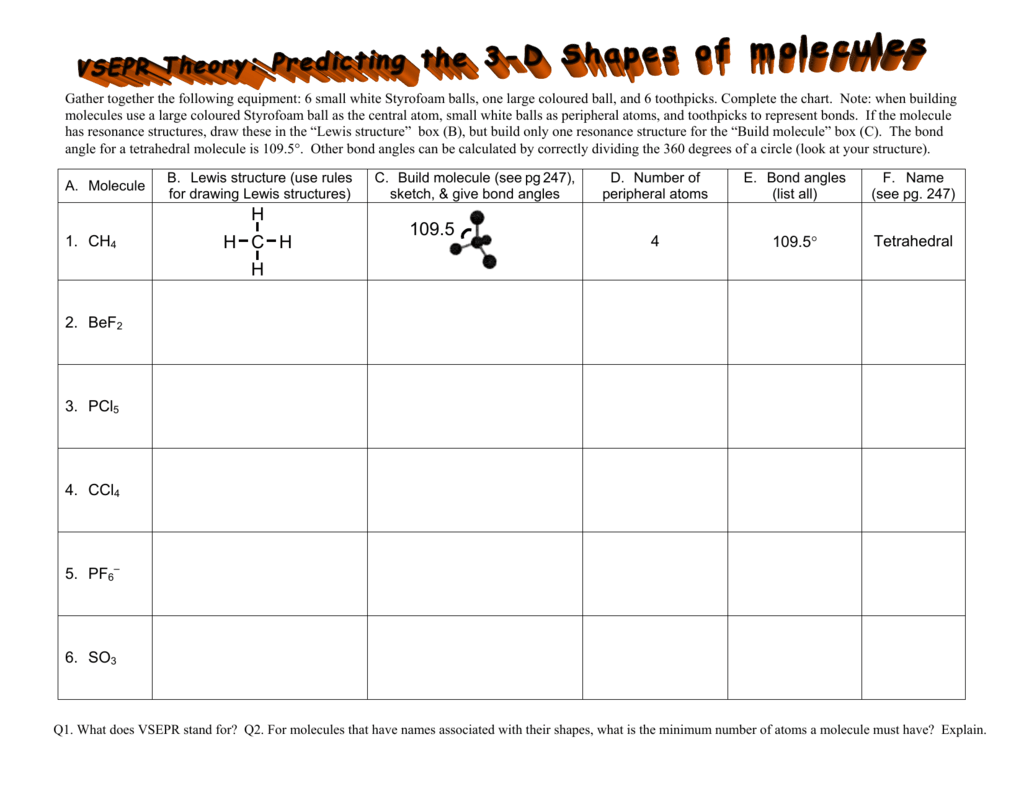
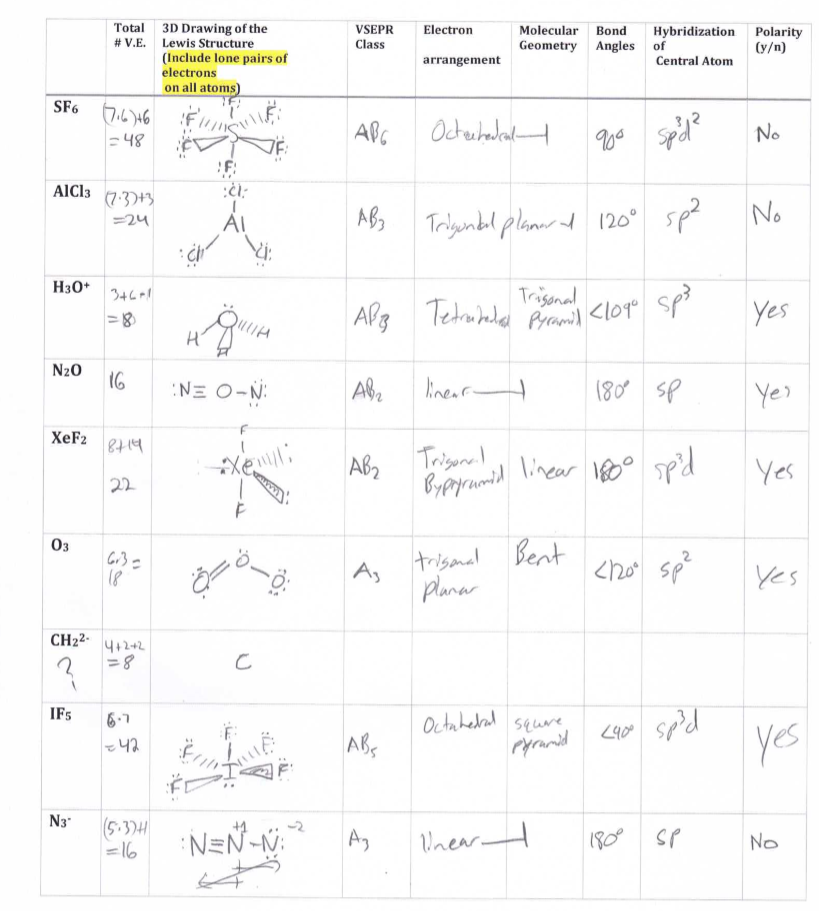
Lewis Structures And Vsepr Worksheet - Web use valence shell electron pair repulsion (vsepr) model to draw and name molecular shapes (bent, linear, trigonal planar, tetrahedral, and trigonal pyramidal). Compounds 1 and 2 are both nonpolar. Polar bonds form between elements with very different electronegativities. Based on the lewis diagrams, which of the following correctly compares the polarities of compounds 1 and 2? Remember, formal charges can sometimes help you decide which is the best lewis dot structure when there is more than one possible structure. Groups of 3 will have 3 packets to turn in). H o h a) how many groups (atoms and lone pairs) surround the central oxygen? Draw the lewis structure for water, h2o. A) bi 3 b) ch 4 c) nf 3 d) c 2 h 2 Web a lewis structure is a dot diagram that shows the valence electrons as well as various bonds present in elements. A) bi 3 b) ch 4 c) nf 3 d) c 2 h 2 Web a lewis structure is a dot diagram that shows the valence electrons as well as various bonds present in elements. Nf3 (a) the total valence electrons = nitrogen + 3*fluorine = 5 + 3*7 = 26 3. Web lewis and vsepr worksheet answers 1. A. Web lewis dot structures and molecule geometries worksheet answer key how to draw a lewis dot structure find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. Bf 3 molecular shape =, bond angle = molecular polarity = r. Nf 3 molecular shape, bond angle =(4 regions; While vsepr is used to draw. Nf 3 molecular shape, bond angle =(4 regions; How does molecule shape change with different numbers of bonds and electron pairs? • identifying electron pair geometries (vsepr). We are given the molecular formulas of a molecule and a polyatomic ion, both conforming to the general formula abn and both having a central atom from the p block of the periodic. (1) draw the lewis structure. Compounds 1 and 2 are both polar. (refer to “note taking guide: Web explore molecule shapes by building molecules in 3d! In the first column of the chart on the next 3 pages, list if the bond is ionic, polar covalent or nonpolar covalent. Tetrahedron c) what is the shape of this molecule (look only at the atoms)? 1 lp) molecular polarity =. Web lewis dot structures and are able to correctly predict the electronic arrangement and molecular geometry before going on to the lab assignment. Draw this vsepr structure next to the lewis structure. Multiple central atoms c 2h 6 c 3h 8. Then, compare the model to real molecules! • identifying electron pair geometries (vsepr). A) bi 3 b) ch 4 c) nf 3 d) c 2 h 2 Expanded octets sf 6 pcl 5 brf 5 xef 4 clf 3 group f: B compounds 1 and 2 are both nonpolar. Draw this vsepr structure next to the lewis structure. Web vsepr worksheet w 318 everett community college tutoring center student support services program 1) briefly describe the primary ideas behind vsepr theory. Web lewis dot structures and molecule geometries worksheet how to draw a lewis dot structure find the total sum of valence electrons that each atom contributes to the. Based on the lewis diagrams, which of the following correctly compares the polarities of compounds 1 and 2? • applying vsepr theory to determine the shapes of molecules. Web • drawing lewis structures for molecules that obey the octet rule. If appropriate, redraw the lewis structure to make it look as close as possible to the molecular shape. Web lewis. Episode 501” or book p. While vsepr is used to draw and name the different molecular shapes like bent, linear, tetrahedral, etc. Web use valence shell electron pair repulsion (vsepr) model to draw and name molecular shapes (bent, linear, trigonal planar, tetrahedral, and trigonal pyramidal). Bf 3 molecular shape =, bond angle = molecular polarity = r. Draw the lewis. We are given the molecular formulas of a molecule and a polyatomic ion, both conforming to the general formula abn and both having a central atom from the p block of the periodic table. Even if you have a burning hatred for gilbert lewis (the guy who came up with these things),. How does molecule shape change with different numbers. Are all five possible electronic geometries represented in this set of molecules? The last part is about vsepr. H o h a) how many groups (atoms and lone pairs) surround the central oxygen? Web • drawing lewis structures for molecules that obey the octet rule. If not, which ones are missing? Web there will be three parts of the experiment. These shapes are determined based off of the lewis structure and the vsepr theory. • applying vsepr theory to determine the shapes of molecules. Draw the lewis structure for water, h2o. 2) for each of the following compounds, a lewis structure, determine the bond angles and molecular shapes for all atoms: Nf3 (a) the total valence electrons = nitrogen + 3*fluorine = 5 + 3*7 = 26 3. If appropriate, redraw the lewis structure to make it look as close as possible to the molecular shape. How does molecule shape change with different numbers of bonds and electron pairs? Nf 3 molecular shape, bond angle =(4 regions; Remember, formal charges can sometimes help you decide which is the best lewis dot structure when there is more than one possible structure. Cs2 (a) total valence electrons = carbon + 2*sulfur = 4 + 2*6 = 16 2. Web lewis structures and vsepr lewis structures worksheet: Compounds 1 and 2 are both nonpolar. Polar bonds form between elements with very different electronegativities. A) bi 3 b) ch 4 c) nf 3 d) c 2 h 2 Compounds 1 and 2 are both polar. Groups of 3 will have 3 packets to turn in). Web the lewis diagrams for the two compounds are shown below. Web • drawing lewis structures for molecules that obey the octet rule. Web explore molecule shapes by building molecules in 3d! Find out by adding single, double or triple bonds and lone pairs to the central atom. Based on the lewis diagrams, which of the following correctly compares the polarities of compounds 1 and 2? Web there will be three parts of the experiment. If appropriate, redraw the lewis structure to make it look as close as possible to the molecular shape. Web vsepr worksheet w 318 everett community college tutoring center student support services program 1) briefly describe the primary ideas behind vsepr theory. Bf 3 molecular shape =, bond angle = molecular polarity = r. You can quickly refer to the periodic table for the group a number for this information. Remember, formal charges can sometimes help you decide which is the best lewis dot structure when there is more than one possible structure. In the first column of the chart on the next 3 pages, list if the bond is ionic, polar covalent or nonpolar covalent. Tetrahedron c) what is the shape of this molecule (look only at the atoms)? Then, compare the model to real molecules!Lewis Dot Structures Worksheet / Lewis Dot Diagram For N Wiring Site
VSEPR Worksheet Lewis Structures, Names, and Bond Angles
Lewis Structure Worksheet with Answers Lewis Structure Notes Worksheet
Lewis Structures And Vsepr Worksheet
Vsepr Worksheet High School Worksheet for Kindergarten
PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram
Download File PDF lewis structure practice worksheet with answers (PDF
Lewis Structures And Vsepr Worksheet
Phet Molecular Shapes Vsepr Activity Worksheet Answers 32 Molecule
Lewis Structures And Vsepr Worksheet
These Shapes Are Determined Based Off Of The Lewis Structure And The Vsepr Theory.
Web Lewis Dot Structures And Are Able To Correctly Predict The Electronic Arrangement And Molecular Geometry Before Going On To The Lab Assignment.
Nf3 (A) The Total Valence Electrons = Nitrogen + 3*Fluorine = 5 + 3*7 = 26 3.
Web Lewis Structures And Vsepr Lewis Structures Worksheet:
Related Post: