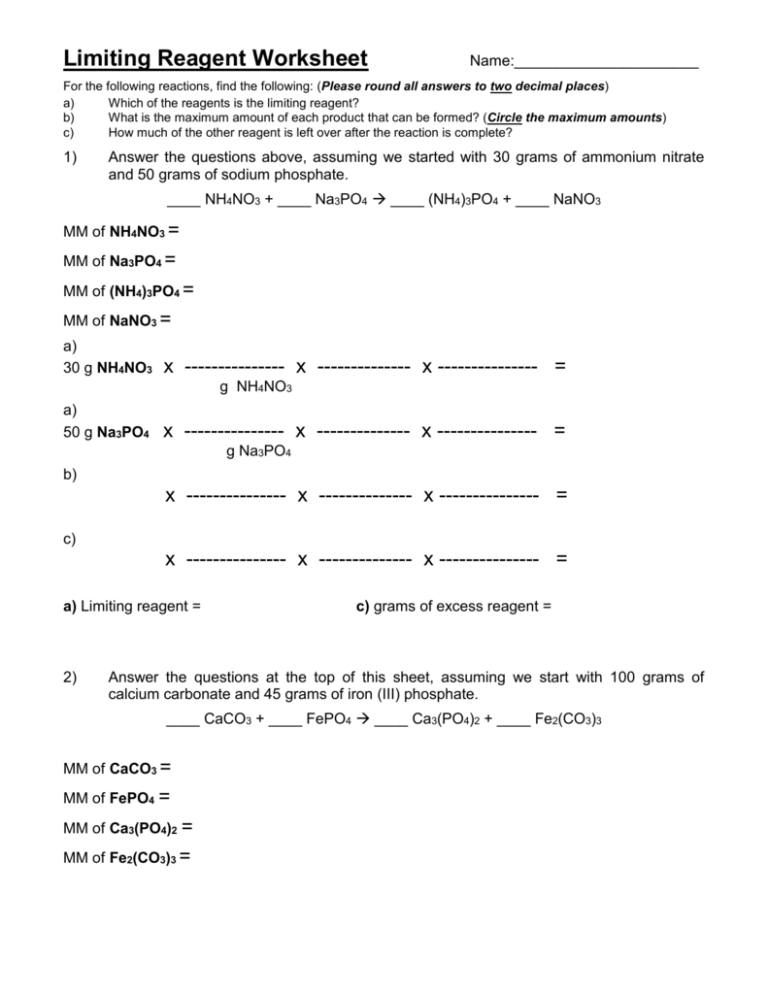
Limiting Reactants Worksheet #1
Limiting Reactants Worksheet #1 - Which of the following is not a step in finding the limiting reactant? Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. Multiplying by the ratio of. So 10/23 = 0.4 moles of hydrogen. Web limiting reagent worksheet all of the questions on this worksheet involve the following reaction: If you start with 14.8 g of c3h8 and 3.44 g of. Web a complete, no prep worksheet that includes 4 problems requiring students to balance or write and balance a chemical equation, then determine the limiting and excess. 1.45 g c 2h 6 1 mol c 2h 6 6 mol h 2o = 0.145 mol h 2o 30.07 g c 2h 6 2 mol c 2h 6 4.50 g o 2 1 mol o 2 6 mol h 2o = 0.121 mol h 2o 31.998 g o 2 7 mol o 2. 6 mol hcl (1 mol al : Web limiting reagent worksheet #1 1. The magnitude of k tells us the relative amounts (concentrations or. Web ii) what percentage yield of iodine was produced. C3h8 + 5o2 ( 3co2 + 4 h2o lr = o2 0.0645 mol co2 1.548 g h2o 13.9 g c3h8 remain problem 2: Web limiting reagent worksheet #1: 1.45 g c 2h 6 1 mol c 2h 6 6 mol. Figuring out how many moles of reactant you have. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and. You start a reaction with. Web limiting reagent worksheet #1 1. So 10/23 = 0.4 moles of hydrogen. 6 mol hcl (1 mol al : Which of the following is not a step in finding the limiting reactant? Now use the moles of the limiting reactant to calculate the mass of the product. Multiply the ratio of product to reactant, or 1:2; The magnitude of k tells us the relative amounts (concentrations or. Convert the 23 grams of sodium to moles; C3h8 + 5o2 ( 3co2 + 4 h2o lr = o2 0.0645 mol co2 1.548 g h2o 13.9 g c3h8 remain problem 2: Web ii) what percentage yield of iodine was produced. Zinc and sulphur react to form zinc sulphide according to the equation. 1.45 g c 2h 6 1 mol c. Web limiting reagent worksheet #1: So 10/23 = 0.4 moles of hydrogen. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and. Figuring out how many moles of reactant you have. Web limiting reagent worksheet all of the questions on this worksheet involve the following reaction: Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. Web (b) step 1 is the slowest step since it depends on both ch 3cho and i 2 2. The magnitude of k tells us the relative amounts (concentrations or. If you start. Which of the following is not a step in finding the limiting reactant? Convert the 23 grams of sodium to moles; C3h8 + 5o2 ( 3co2 + 4 h2o lr = o2 0.0645 mol co2 1.548 g h2o 13.9 g c3h8 remain problem 2: Web now look at the ratio of the reactants: Web (b) step 1 is the slowest. 6 mol hcl (1 mol al : So 0.4 (1/2) = 0.2 moles of hydrogen. Web limiting reagent worksheet #1: Which of the following is not a step in finding the limiting reactant? Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. Web limiting reagent worksheet #1 1. C3h8 + 5o2 ( 3co2 + 4 h2o lr = o2 0.0645 mol co2 1.548 g h2o 13.9 g c3h8 remain problem 2: Convert the 23 grams of sodium to moles; Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent. Web (b) step 1 is the slowest step since it depends on both ch 3cho and i 2 2. So 10/23 = 0.4 moles of hydrogen. Balance the equation first) c3h8 + o2 g co2 + h2o. Now use the moles of the limiting reactant to calculate the mass of the product. So 0.4 (1/2) = 0.2 moles of hydrogen. 6 mol hcl (1 mol al : Now use the moles of the limiting reactant to calculate the mass of the product. So 10/23 = 0.4 moles of hydrogen. Web ii) what percentage yield of iodine was produced. Web limiting reagent worksheet all of the questions on this worksheet involve the following reaction: So 0.4 (1/2) = 0.2 moles of hydrogen. The magnitude of k tells us the relative amounts (concentrations or. Web a complete, no prep worksheet that includes 4 problems requiring students to balance or write and balance a chemical equation, then determine the limiting and excess. 1.45 g c 2h 6 1 mol c 2h 6 6 mol h 2o = 0.145 mol h 2o 30.07 g c 2h 6 2 mol c 2h 6 4.50 g o 2 1 mol o 2 6 mol h 2o = 0.121 mol h 2o 31.998 g o 2 7 mol o 2. Web now look at the ratio of the reactants: Which of the following is not a step in finding the limiting reactant? You start a reaction with. Multiplying by the ratio of. Figuring out how many moles of reactant you have. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. Web (b) step 1 is the slowest step since it depends on both ch 3cho and i 2 2. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and. If you start with 14.8 g of c3h8 and 3.44 g of. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. Web limiting reagent worksheet #1 1. You start a reaction with. Some of the worksheets for this concept are limiting reagent work, practice problems limiting. So 0.4 (1/2) = 0.2 moles of hydrogen. Convert the 23 grams of sodium to moles; Balance the equation first) c3h8 + o2 g co2 + h2o. Web ii) what percentage yield of iodine was produced. Web limiting reagent worksheet all of the questions on this worksheet involve the following reaction: So 10/23 = 0.4 moles of hydrogen. Web (b) step 1 is the slowest step since it depends on both ch 3cho and i 2 2. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and. 1.45 g c 2h 6 1 mol c 2h 6 6 mol h 2o = 0.145 mol h 2o 30.07 g c 2h 6 2 mol c 2h 6 4.50 g o 2 1 mol o 2 6 mol h 2o = 0.121 mol h 2o 31.998 g o 2 7 mol o 2. Zinc and sulphur react to form zinc sulphide according to the equation. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. Multiply the ratio of product to reactant, or 1:2; Multiplying by the ratio of. The magnitude of k tells us the relative amounts (concentrations or.Limiting Reagent Worksheet 1 YouTube
Limiting Reagent Worksheet 1 Answers trendstoday
42 limiting reactant worksheet answers Worksheet Resource
Stoichiometry Limiting Reagent Worksheet Answers —
Limiting Reagent Worksheet 1 worksheet
Worksheet On Limiting Reactants
Limiting Reactant Wkst 1 Chemistry Worksheet Limiting Reactant
Limiting Reagent Worksheet 1 Answers trendstoday
Limiting Reagent Worksheet 1
Chemistry Worksheet Limiting Reactant Worksheet 1
Web Now Look At The Ratio Of The Reactants:
6 Mol Hcl (1 Mol Al :
Figuring Out How Many Moles Of Reactant You Have.
Now Use The Moles Of The Limiting Reactant To Calculate The Mass Of The Product.
Related Post: