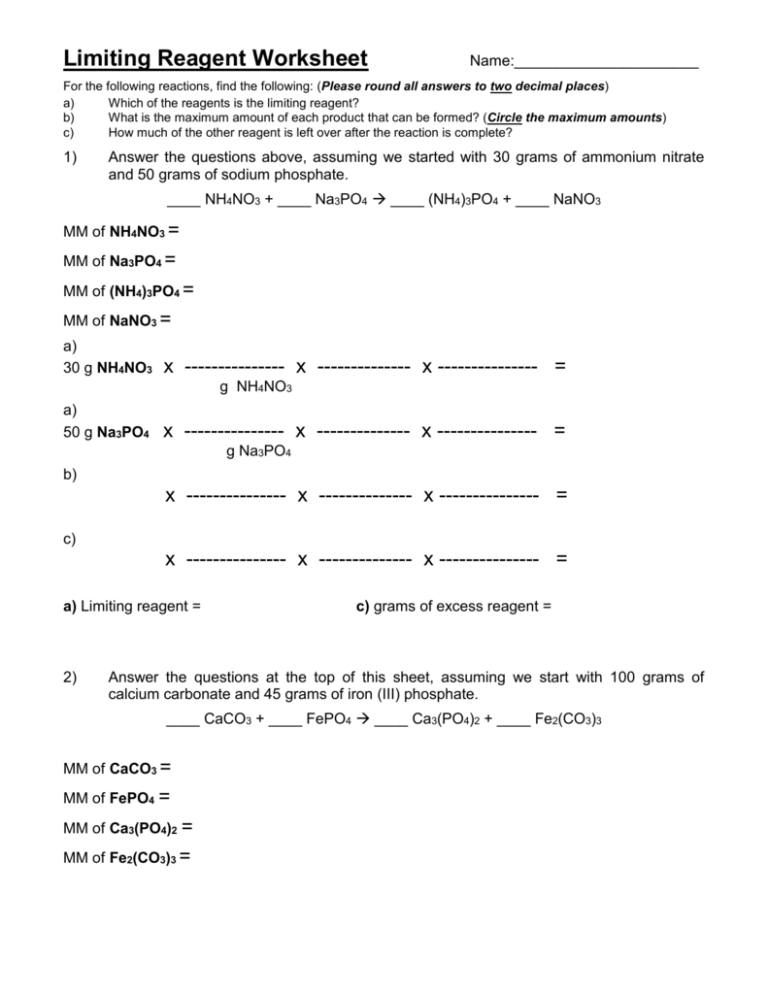
Limiting Reagent Worksheet Answer Key
Limiting Reagent Worksheet Answer Key - Balance the equation first) c 3 h 8 + o 2 g co 2 + h 2 o. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and. Nh 3 + o 2 no + h 2 o. In an experiment, 3.25 g of nh 3 are allowed to react with 3.50 g of o 2. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. C3h8+ o2→ co2+ h2o a. When copper (ii) chloride reacts with sodium nitrate, copper (ii). 2no(g) + o2 ( 2no2 in one experiment 0.866 mol of. Limiting reagent worksheet #1 1. Web some of the worksheets for this concept are limiting reagent work, limiting reagent work, limiting reagents, limiting reagent practice problems, work limiting reactants. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. Web limiting reagent worksheet all of the questions on this worksheet involve the following reaction: Web limiting reagent worksheet #1 1. Number of moles mass given. Web limiting reagent worksheet #1: Limiting reagent worksheet #1 1. Web limiting reagent worksheet #1 1. Balance the equation first) c 3 h 8 + o 2 g co 2 + h 2 o. If you start with 14.8 g of c 3 h 8 and 3.44. Web limiting reagent worksheet #2 1. Which chemical is the limiting. All of the questions on this worksheet involve the following reaction: 2no(g) + o2 ( 2no2 in one experiment 0.866 mol of. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. Web limiting reagent worksheet #1 1. Web limiting reagents worksheet 1. 2no(g) + o2 ( 2no2 in one experiment 0.866 mol of. Web limiting reagent worksheet all of the questions on this worksheet involve the following reaction: All of the questions on this worksheet involve the following reaction: Nh 3 + o 2 no + h 2 o. We can calculate the limiting reagent in a reaction by many factors, but which of the factors cannot help to determine the limiting reactant: Web limiting reagent worksheet all of the questions on this worksheet involve the following reaction: When copper (ii) chloride reacts with sodium nitrate, copper (ii). Limiting reagent worksheet #1 1. When copper (ii) chloride reacts with. Which chemical is the limiting. All of the questions on this worksheet involve the following reaction: Number of moles mass given. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. Web limiting reagent worksheet all of the questions on this worksheet involve. In an experiment, 3.25 g of nh 3 are allowed to react with 3.50 g of o 2. Balance the equation first) c 3 h 8 + o 2 g co 2 + h 2 o. Web limiting reagent worksheet #1 1. 2no(g) + o2 ( 2no2 in one experiment 0.866 mol of. Web limiting reagent worksheet #1 1. C3h8+ o2→ co2+ h2o a. Balance the equation first) c 3 h 8 + o 2 g co 2 + h 2 o. When copper (ii) chloride reacts with sodium nitrate, copper (ii). Web limiting reagent worksheet #1 1. Nh 3 + o 2 no + h 2 o. C3h8+ o2→ co2+ h2o a. We can calculate the limiting reagent in a reaction by many factors, but which of the factors cannot help to determine the limiting reactant: All of the questions on this worksheet involve the following reaction: Web limiting reagent worksheet #1 1. Limiting reagents (answer key) take the reaction: Limiting reagent worksheet #1 1. Limiting reagents (answer key) take the reaction: Number of moles mass given. Which chemical is the limiting. When copper (ii) chloride reacts with sodium nitrate, copper (ii). 2no(g) + o2 ( 2no2 in one experiment 0.866 mol of. Nh 3 + o 2 no + h 2 o. Nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: Which chemical is the limiting. Web limiting reagent worksheet #1 1. Number of moles mass given. We can calculate the limiting reagent in a reaction by many factors, but which of the factors cannot help to determine the limiting reactant: All of the questions on this worksheet involve the following reaction: Web some of the worksheets for this concept are limiting reagent work, limiting reagent work, limiting reagents, limiting reagent practice problems, work limiting reactants. Web limiting reagent worksheet all of the questions on this worksheet involve the following reaction: Web limiting reagents worksheet 1. In an experiment, 3.25 g of nh 3 are allowed to react with 3.50 g of o 2. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and. Limiting reagent worksheet #1 1. When copper (ii) chloride reacts with sodium nitrate, copper (ii). Web limiting reagent worksheet #2 1. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a reaction. If you start with 14.8 g of c 3 h 8 and 3.44. C3h8+ o2→ co2+ h2o a. Web limiting reagent worksheet #1: Web limiting reagents worksheet 1. C3h8 + 5o2 ( 3co2 + 4 h2o lr = o2 0.0645 mol co2 1.548 g h2o 13.9 g c3h8 remain problem 2: C3h8+ o2→ co2+ h2o a. All of the questions on this worksheet involve the following reaction: When copper (ii) chloride reacts with sodium nitrate, copper (ii). When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and. Web limiting reagent worksheet all of the questions on this worksheet involve the following reaction: Web limiting reagent worksheet #1: Nh 3 + o 2 no + h 2 o. If you start with 14.8 g of c 3 h 8 and 3.44. Web limiting reagent worksheet #1 1. Web some of the worksheets for this concept are limiting reagent work, limiting reagent work, limiting reagents, limiting reagent practice problems, work limiting reactants. 2no(g) + o2 ( 2no2 in one experiment 0.866 mol of. Balance the equation first) c 3 h 8 + o 2 g co 2 + h 2 o. We can calculate the limiting reagent in a reaction by many factors, but which of the factors cannot help to determine the limiting reactant: In an experiment, 3.25 g of nh 3 are allowed to react with 3.50 g of o 2.Limiting Reagents And Percentage Yield Worksheet Answers
Limiting Reactant And Percent Yield Practice Worksheet Answer Key
Limiting Reactant Worksheet Answers
Limiting Reagent Worksheet Answers Chemical Reactions Sodium
moles worksheet answers with work
Limiting Reactant And Percent Yield Practice Worksheet Answer Key
Limiting Reagent Worksheet 1 Answers trendstoday
42 limiting reactant worksheet answers Worksheet Resource
Limiting Reagent Worksheet Answer Key Alphabet Worksheets
Carrying Capacity And Limiting Factors Worksheet 1 Answer Key
Which Chemical Is The Limiting.
Limiting Reagents (Answer Key) Take The Reaction:
Nitric Oxide (No) Reacts With Oxygen Gas To Form Nitrogen Dioxide (No2), A Dark Brown Gas:
Web Limiting Reagent Worksheet #2 1.
Related Post: