Molality M Worksheet Answers
Molality M Worksheet Answers - Solve for mol in the latter (mol =g/mm) and plug it into the former to get m =. 1 liter = 1000 mls 2.5 moles of sodium chloride is dissolved to make. 4.0 moles of licl is dissolved in 5.0 liters of. Select the correct relation between molarity and molality. (x) (1.00 l) = 245.0 g / 98.08 g mol¯1; A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution is prepared with a total. Calculate the molarity of a 0.4 in 0.250 l solution. Web you can also take the definition of molarity, m = mol/l, and the definition of molar mass, mm=g/mol. Web find the molarity (concentration) of the following solutions: Web molarity = moles of solute / liters of solution (abbreviation = m) molality = moles of solute / kg of solvent (abbreviation = m) normality = number of equivalent of solute x molarity of. Sol 101k 3 moles c6h1206 in 0.1 l equals what molarit ? X = 0.125 m 4. Select the correct relation between molarity and molality. Web this quiz and worksheet allow students to test the following skills: The amount of compound dissolved in a solution can be calculated and expressed numerically. Web you can also take the definition of molarity, m = mol/l, and the definition of molar mass, mm=g/mol. Calculate the molarity of a 0.4 in 0.250 l solution. X = 0.479 m 2. Describes calculations involving molal solutions. Molarity answer key part 1 molarity: A quantitative description of solution concentration. 4.0 moles of licl is dissolved in 5.0 liters of. X = 0.479 m 2. Start with the definition of molality. Web molality worksheet what is the molality of a solution that contains 63.0 g hno3 in 0.500 kg h2o? (x) (1.00 l) = 245.0 g / 98.08 g mol¯1; X = 0.479 m 2. How many moles of ki are present in. Web molality worksheet what is the molality of a solution that contains 63.0 g hno3 in 0.500 kg h2o? (x) (0.4000 l) = 5.30 g / 106.0 g mol¯1; Web this worksheet provides many examples for students to practice calculations involving molarity & X = 2.498 m 3. Web molarity = moles of solute / liters of solution (abbreviation = m) molality = moles of solute / kg of solvent (abbreviation = m) normality = number of equivalent of solute x molarity of. Web how is molality different? A. Calculate the molarity of a 0.4 in 0.250 l solution. Web this worksheet provides many examples for students to practice calculations involving molarity & Molality is the number of moles of solute per kilogram of solvent. Abbreviated m molarity = moles of solute ÷ liters of solution problems:. A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution. Web up to 24% cash back molarity (m) = m oles of solute molality (m or 𝜇 ) = m oles of solute liters of solvent kg of solvent molarity example: How many moles of ki are present in. Calculate the molarity of a 0.4 in 0.250 l solution. Molarity = mole/liters volume must be in liters! Select the correct. Solve for mol in the latter (mol =g/mm) and plug it into the former to get m =. X = 0.125 m 4. Web this quiz and worksheet allow students to test the following skills: (x) (0.4000 l) = 5.30 g / 106.0 g mol¯1; X = 2.498 m 3. 1 liter = 1000 mls 2.5 moles of sodium chloride is dissolved to make. X = 2.498 m 3. (x) (1.00 l) = 28.0 g / 58.45 g mol¯1; Molarity = mole/liters volume must be in liters! Web molality worksheet what is the molality of a solution that contains 63.0 g hno3 in 0.500 kg h2o? Web you can also take the definition of molarity, m = mol/l, and the definition of molar mass, mm=g/mol. (x) (0.4000 l) = 5.30 g / 106.0 g mol¯1; Web this quiz and worksheet allow students to test the following skills: Web this worksheet provides many examples for students to practice calculations involving molarity & 4.0 moles of licl is. Calculate the molarity of a 0.4 in 0.250 l solution. What is the molality of a solution that contains.500 mol hc2h3o2 in 0.125 kg. Select the correct relation between molarity and molality. Web this worksheet provides many examples for students to practice calculations involving molarity & Web find the molarity (concentration) of the following solutions: (x) (0.4000 l) = 5.30 g / 106.0 g mol¯1; A complete answer key is provided at the end. Web answers m 1 v 1 = m 2 v 2 (1.71 m) (25.0 ml) = m 2 (65.0 ml) m 2 = 0.658 m m = mol/l = (25.0/40.0) / (0.325) = 1.92 mol/l g = (m) (l) (fw) = (0.400) ( (0.225) (119) = 10.7 g. Web this quiz and worksheet allow students to test the following skills: X = 0.125 m 4. Start with the definition of molality. 1 liter = 1000 mls 2.5 moles of sodium chloride is dissolved to make. A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution is prepared with a total. Sol 101k 3 moles c6h1206 in 0.1 l equals what molarit ? Web you can also take the definition of molarity, m = mol/l, and the definition of molar mass, mm=g/mol. Solve for mol in the latter (mol =g/mm) and plug it into the former to get m =. Molarity = mole/liters volume must be in liters! Web how is molality different? X = 2.498 m 3. Web up to 24% cash back molarity (m) = m oles of solute molality (m or 𝜇 ) = m oles of solute liters of solvent kg of solvent molarity example: X = 0.125 m 4. The amount of compound dissolved in a solution can be calculated and expressed numerically. (x) (1.00 l) = 245.0 g / 98.08 g mol¯1; 4.0 moles of licl is dissolved in 5.0 liters of. What is the molality of a solution that contains.500 mol hc2h3o2 in 0.125 kg. Web this quiz and worksheet allow students to test the following skills: Web how is molality different? A complete answer key is provided at the end. Sol 101k 3 moles c6h1206 in 0.1 l equals what molarit ? Molarity = mole/liters volume must be in liters! Start with the definition of molality. Web molarity (m) moles of solute / liter of solution (most commonly used unit of concentration) molality (m) moles of solute / kg of solvent compare to molarity and notice differences in. Calculate the molarity of a 0.4 in 0.250 l solution. Molality is the number of moles of solute per kilogram of solvent. Describes calculations involving molal solutions. Molarity answer key part 1 molarity:Molarity Worksheet Answer Key
Molarity Practice Worksheet Answer
️Molarity Practice Worksheet Free Download Goodimg.co
molarity m worksheet answers
Molarity Practice Worksheet Answer
Molarity Practice Worksheet Answer
Molarity Worksheet Answer Key
Molarity Worksheet 1 Answer Key Chemistry
Molarity Worksheet Answer Key
molarity m worksheet answers
(X) (1.00 L) = 28.0 G / 58.45 G Mol¯1;
Select The Correct Relation Between Molarity And Molality.
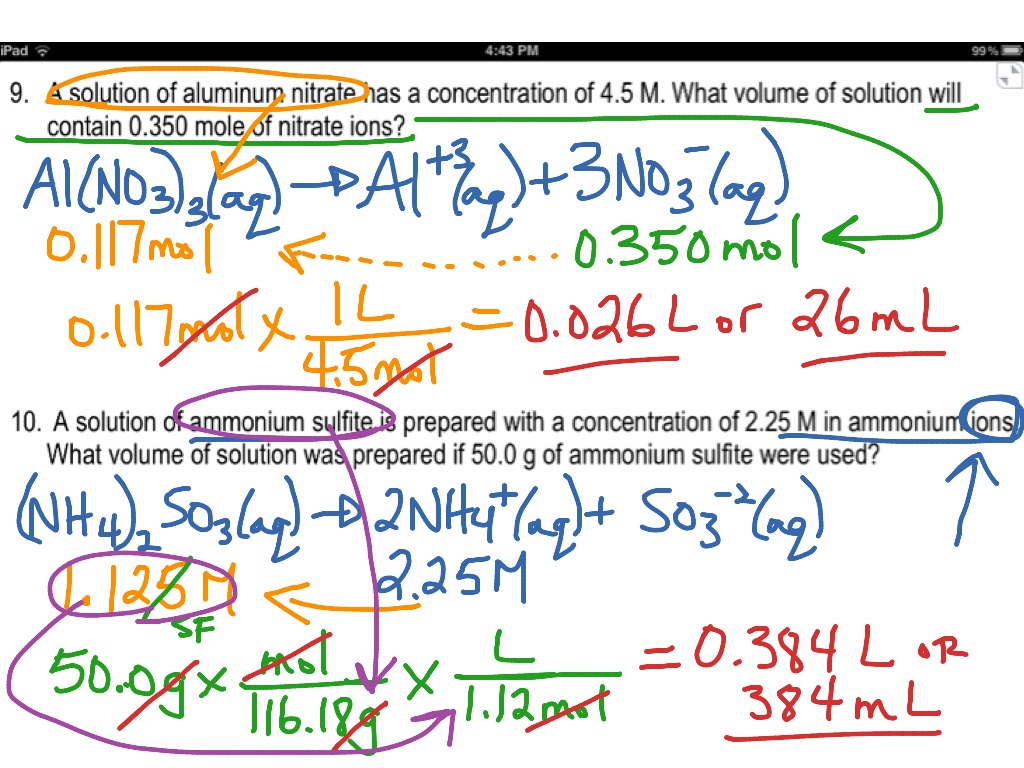
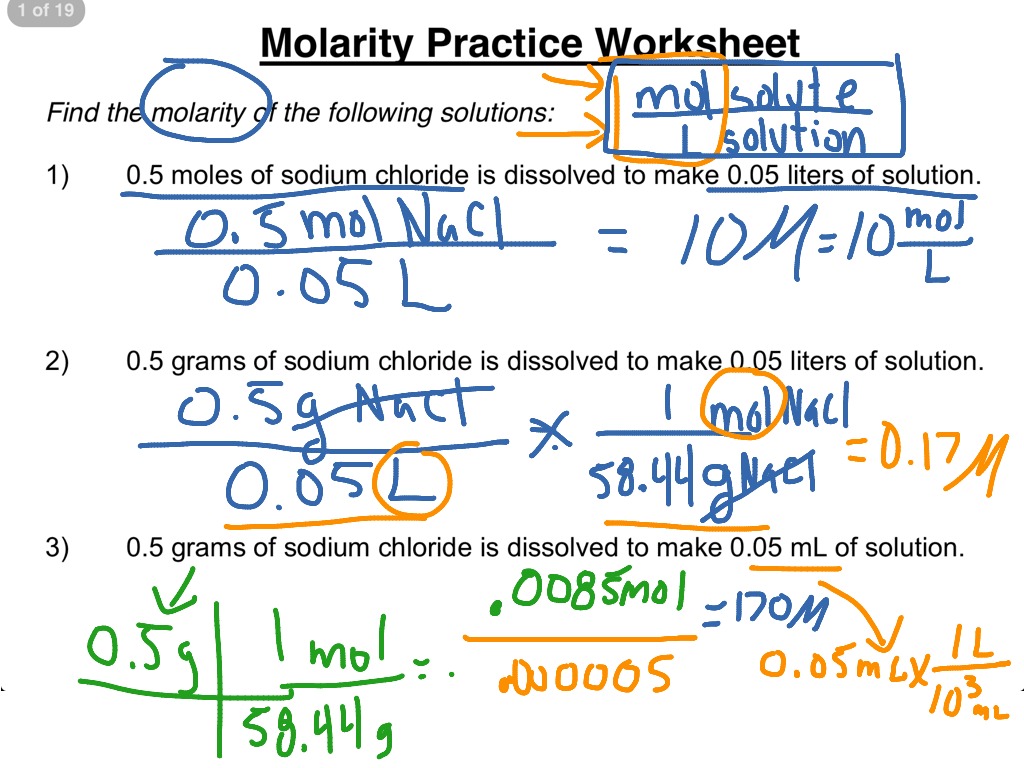
Web Find The Molarity (Concentration) Of The Following Solutions:
Web Molality Worksheet What Is The Molality Of A Solution That Contains 63.0 G Hno3 In 0.500 Kg H2O?
Related Post: