Molarity By Dilution Worksheet Answers
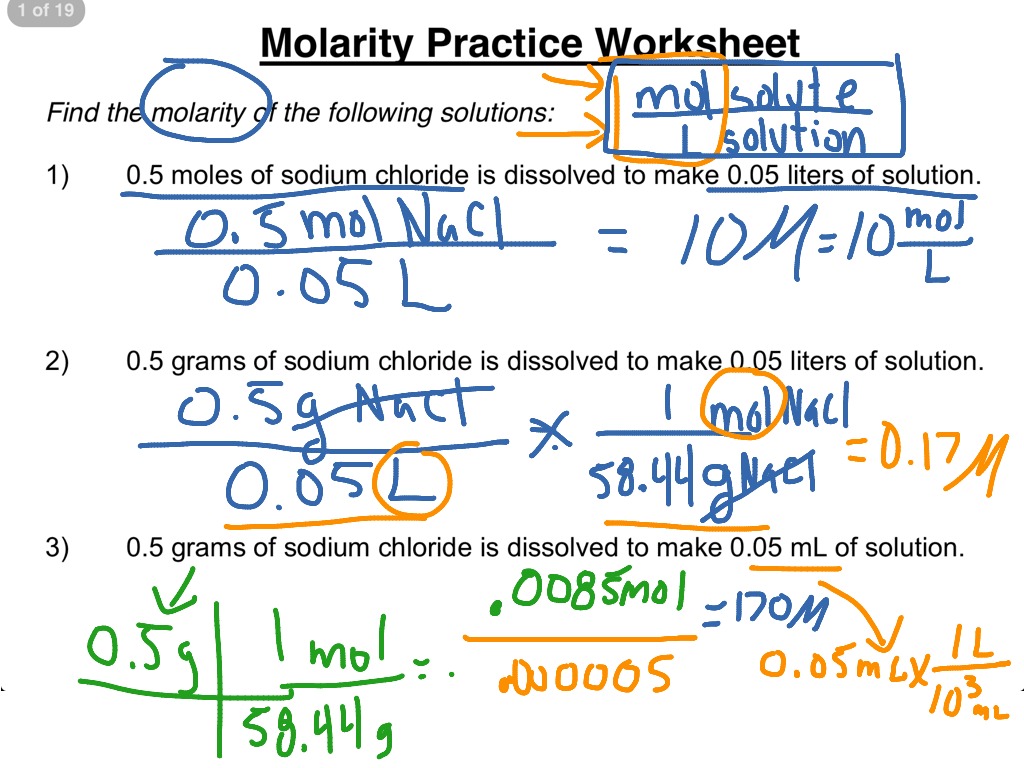
Molarity By Dilution Worksheet Answers - Web m = moles of solute / liters of solution and mv = grams / molar mass. Web solutions worksheet molarity and dilution problems answer key march 25, 2023 by tamble it’s not only about getting the right answers; Web m2 and v2 refer to the molarity and volume of the lab solution. 2) if i add water to 100 ml of a 0.15 m naoh. Using the dilution equation, we. Web you should try to answer the questions without referring to your textbook. Web molarity practice worksheet find the molarity of the following solutions: The correct option is ‘a’. What is the concentration of each ion in solution #1? Make sure you pay close attention to multiply and divide. What is the concentration of each ion in solution #1? 1) 176.4 g nahco3 2) 42.5 g nano3 3) 221.76 g ccl4 4) 120 ml 1.0 m solution + 180 ml water 5) 125 ml 2.0m solution + 375 ml of water 6) 4.8 m 7) 127.5 ml 4. 4) 0.5 moles of sodium chloride is dissolved to make 0.05. Note that the 58.45 is in the denominator on the right side and in the final answer you will have 0.2 times 0.1 times 58.45. 2) if i add water to 100 ml of a 0.15 m naoh. Web molarity practice worksheet find the molarity of the following solutions: Using the dilution equation, we. Web the molarity of this solution. How much solute is contained in 500. Ml of a 2.5 m solution?. When a soluble ionic compound. Web molarity practice worksheet find the molarity of the following solutions: Note that the 58.45 is in the denominator on the right side and in the final answer you will have 0.2 times 0.1 times 58.45. A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution is prepared with a total volume of 0.0750\,\text {l} 0.0750l. When a soluble ionic compound. Web the molarity of this solution is: Web solutions worksheet molarity and dilution problems answer key march 25, 2023 by tamble it’s not only about getting the right answers; Web m2 and v2. Web solutions worksheet molarity and dilution problems answer key march 25, 2023 by tamble it’s not only about getting the right answers; Web the molarity of this solution is: Web molarity practice worksheet find the molarity of the following solutions: Molarity of a solution (m) = n/v. A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution is. Typically, the solution is for the molarity (m). Molarity of a solution (m) = n/v. For example, look at answer #8. How many ml of 0.800 m can you make? Calculate molarity if 25.0 ml of 1.75 m hcl diluted to. Using the dilution equation, we. Ml of a 2.5 m solution?. For example, look at answer #8. Web solutions worksheet molarity and dilution problems answer key march 25, 2023 by tamble it’s not only about getting the right answers; 4) 0.5 moles of sodium chloride is dissolved to make 0.05 liters of solution. If you get stuck, try asking another group for help. Web molarity is defined as the number of moles of solute in exactly 1 liter (1 l) of the solution: Web up to 24% cash back (molarity)? Web solutions worksheet molarity and dilution problems answer key march 25, 2023 by tamble it’s not only about getting the right answers; Web. For example, look at answer #8. First write an equation) 3. Web m = moles of solute / liters of solution and mv = grams / molar mass. 1) 176.4 g nahco3 2) 42.5 g nano3 3) 221.76 g ccl4 4) 120 ml 1.0 m solution + 180 ml water 5) 125 ml 2.0m solution + 375 ml of water. Web solutions worksheet molarity and dilution problems answer key march 25, 2023 by tamble it’s not only about getting the right answers; Note that the 58.45 is in the denominator on the right side and in the final answer you will have 0.2 times 0.1 times 58.45. Web molarity practice worksheet find the molarity of the following solutions: Make sure. A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution is prepared with a total volume of 0.0750\,\text {l} 0.0750l. Forming aqueous solutions from ionic solids many ionic compounds are soluble in water. If you get stuck, try asking another group for help. Make sure you pay close attention to multiply and divide. What is the concentration of each ion in solution #1? Molarity of a solution (m) = n/v. Web m2 and v2 refer to the molarity and volume of the lab solution. How many ml of 0.800 m can you make? 1) 176.4 g nahco3 2) 42.5 g nano3 3) 221.76 g ccl4 4) 120 ml 1.0 m solution + 180 ml water 5) 125 ml 2.0m solution + 375 ml of water 6) 4.8 m 7) 127.5 ml 4. Web molarity practice worksheet find the molarity of the following solutions: Rearranging the formula to make 'v' the subject allows. M1v1 = m2v2 (0.15 m)(125 ml) = x (150 ml) = 0.125 m Typically, the solution is for the molarity (m). Using the dilution equation, we. Ml of a 2.5 m solution?. Web you should try to answer the questions without referring to your textbook. Web dilutions worksheet 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 2) if i add water to 100 ml of a 0.15 m naoh. How much solute is contained in 500. Web example #1 53.4 ml of a 1.50 m solution of nacl is on hand, but you need some 0.800 m solution. 1) 176.4 g nahco3 2) 42.5 g nano3 3) 221.76 g ccl4 4) 120 ml 1.0 m solution + 180 ml water 5) 125 ml 2.0m solution + 375 ml of water 6) 4.8 m 7) 127.5 ml 4. Web m = moles of solute / liters of solution and mv = grams / molar mass. Ml of a 2.5 m solution?. First write an equation) 3. Web the molarity of this solution is: Rearranging the formula to make 'v' the subject allows. What is the concentration of each ion in solution #1? Web m2 and v2 refer to the molarity and volume of the lab solution. For our purposes, we will rearrange the formula to tell us how much of the stock solution to use to create the. Make sure you pay close attention to multiply and divide. Web molarity is defined as the number of moles of solute in exactly 1 liter (1 l) of the solution: Web you should try to answer the questions without referring to your textbook. Calculate molarity if 25.0 ml of 1.75 m hcl diluted to. Web up to 24% cash back (molarity)? If you get stuck, try asking another group for help. How much solute is contained in 500.️Molarity Practice Worksheet Free Download Goodimg.co
Molarity And Dilution Worksheet
molarity dilution worksheet
Molarity Worksheet Answer Key
Molarity Worksheet 1 Answer Key Chemistry
7 Molarity Worksheet With Answers /
__LINK__ Molarity By Dilution Worksheet Answers Chemistry If8766
molarity dilution worksheet
dilution worksheet chemistry
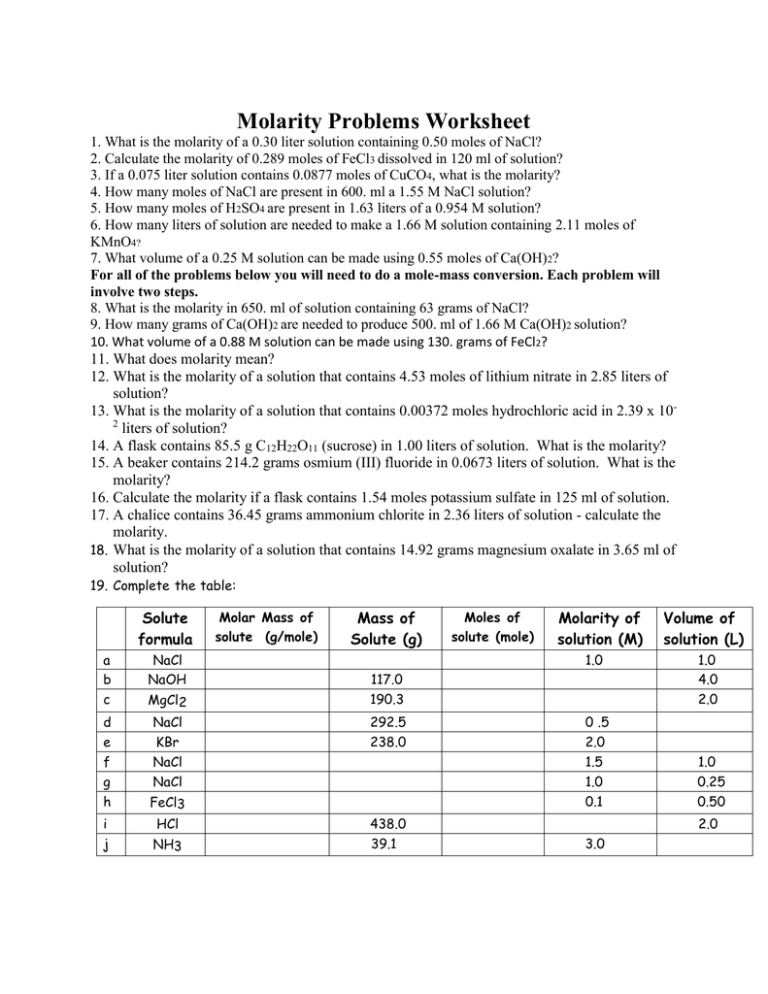
Molarity Problems Worksheet
Using The Dilution Equation, We.
For Example, Look At Answer #8.
2) If I Add Water To 100 Ml Of A 0.15 M Naoh.
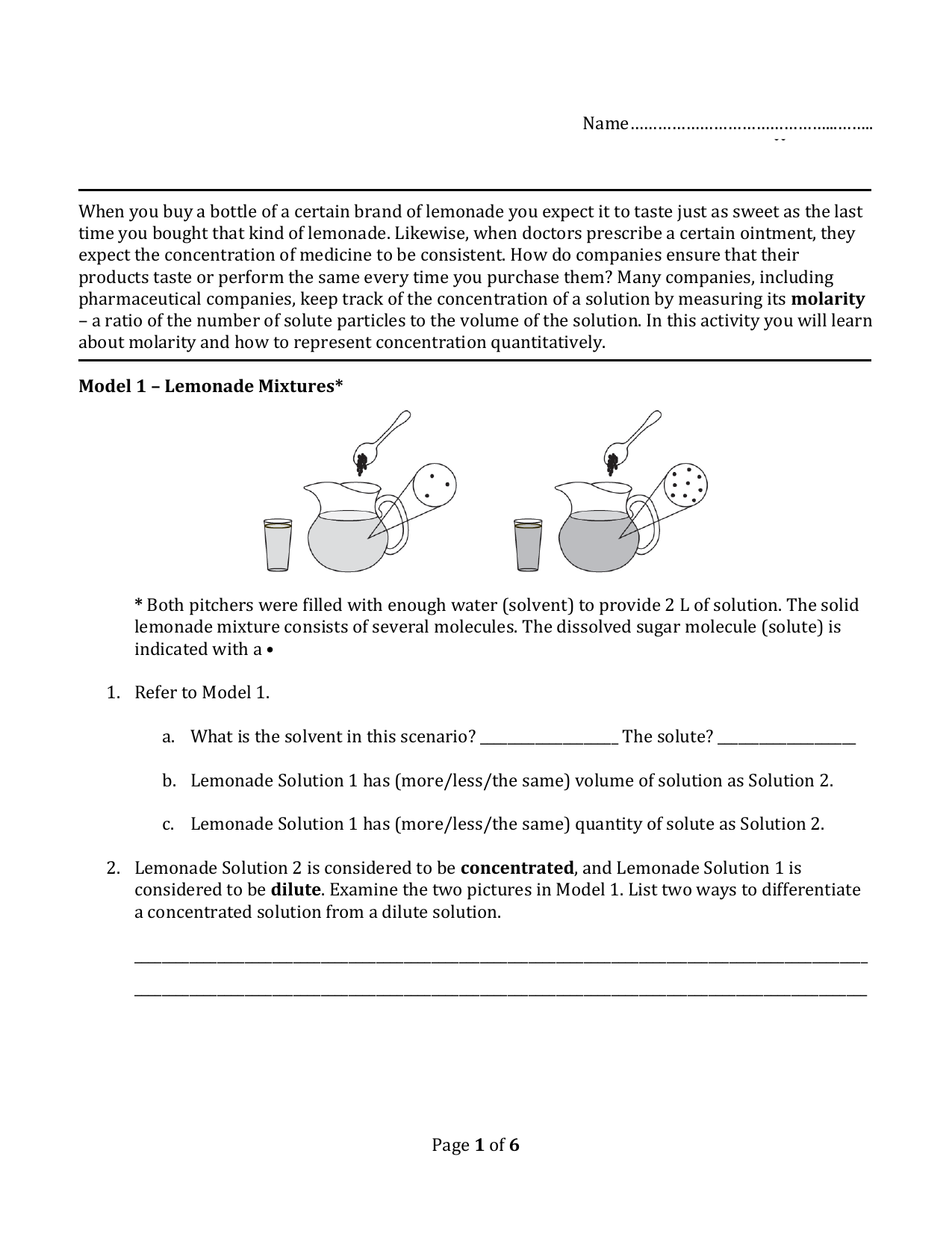
When A Soluble Ionic Compound.
Related Post: