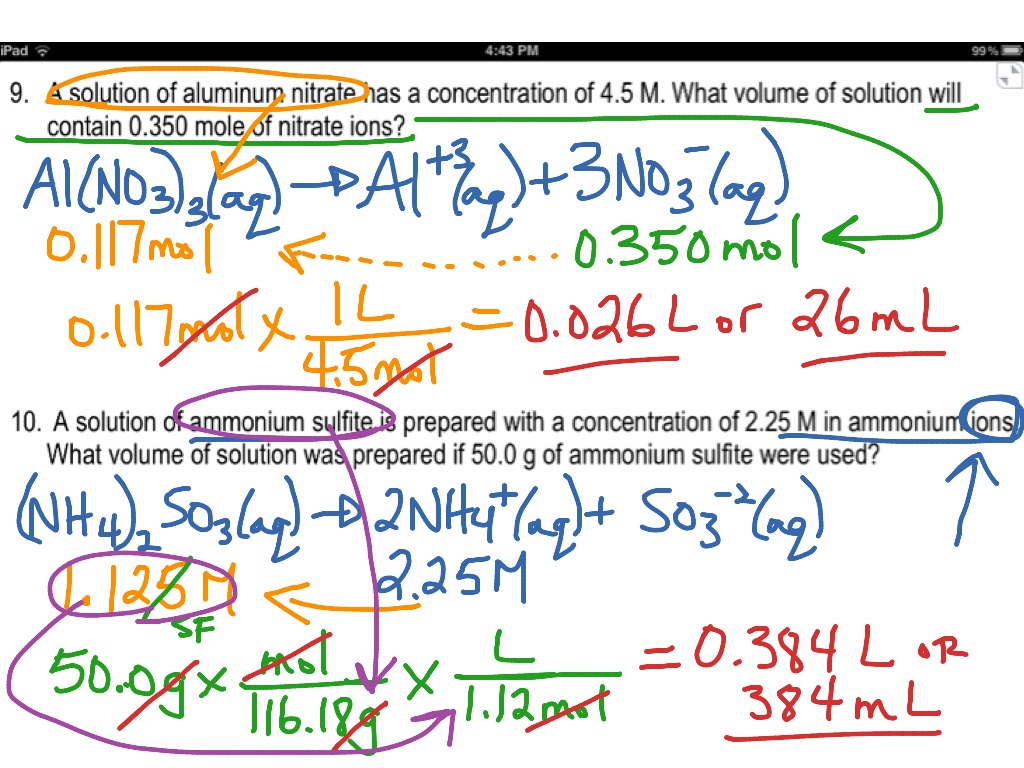
Molarity Practice Worksheet Answer Key
Molarity Practice Worksheet Answer Key - Zn (no 3) 2 alcl 3 agbr fepo 4 cuac 2. Determine the molarity of a solution containing 2 mol of ki in 140 ml total volume of solution ans: Such mixture is called a solution. When we dissolve a cube of sugar in one cup of water, we create a homogeneous mixture. Solutions to the molarity practice worksheet for the first five problems, you need to use the equation that says that the molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Calculate ml of 0.650m kno 3 needed to contain 25.0g kno 3. 1) 1.2 m 2) 1.4 m 3) 0.11 m 4) 0.66 mol 5) 0.0075 mol 6) 0.286 l 7) 0.015 mol 8) 144 g 9) 1270 molarity extra practice worksheet 1. Abbreviated m molarity =moles of solute ÷ liters of solution problems: Ml of solution made from 0.70 moles of licl and water? 1) 1 moles of potassium fluoride is dissolved to make 0 l of solution. What is the molarity of 500. What is the concentration of a solution of nacl if 40 ml of a 2 m nacl solution is diluted to a total volume of 500 ml? Calculate grams of solute needed to prepare 225 ml of 0.400 m kbr solution. Zn (no 3) 2 alcl 3 agbr fepo 4 cuac 2. Web calculate. 1 mole kf = 10. Web solutions molarity worksheet name: Calculate ml of 0.650m kno 3 needed to contain 25.0g kno 3. Given that the molar mass of k2co3 is 138.21 g/mol, this means that we'll require 97 grams 2. 724.4 g of ammonium phosphate in 4500 ml of alcohol. 1 mole kf = 10. Web answer key included!check out all my resources for solutions:factors affecting solubility labsolutions vocab slidessolubility curve (graphs) notes & slidessolution vocabulary dominossolubility curves graphing & questionsgas solubility mini labsolutions review stations subjects: 724.4 g of ammonium phosphate in 4500 ml of alcohol. Determine the molarity of a solution containing 2 mol of ki in 140. How many moles of sucrose are dissolved in 250 ml of solution if the solution concentration is 0.150 m? 69.1 grams 2) how many liters of 4 m solution can be made using 100 grams of lithium bromide? 0.5 grams of sodium chloride is dissolved to make 0.05 liters of solution. Web this worksheet provides many examples for students to. 724.4 g of ammonium phosphate in 4500 ml of alcohol. We may do this by multiplying both sides of the equation by the l solution. 3.47 l 3) what is the concentration of an aqueous solution with a volume of 450 ml that contains 200 grams of iron (ii. Calculate the molarity of a solution which contains 0.40 mol of. Such mixture is called a solution. (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the average concentration of cholesterol in human serum (b) 4.25 g of nh 3 in 0.500 l of solution, the concentration of nh 3 in household ammonia 724.4 g of ammonium phosphate in 4500 ml of alcohol. Calculate the. Web calculate molarity by dissolving 25.0g naoh in 325 ml of solution. (a) 0.195 g of cholesterol, c 27 h 46 o, in 0.100 l of serum, the average concentration of cholesterol in human serum (b) 4.25 g of nh 3 in 0.500 l of solution, the concentration of nh 3 in household ammonia A complete answer key is provided. Moles of solute = 2 moles of sugar. 1 g kf x 1 mole kf = 0 mol kf 0 mol kf = 0 m 58 g kf 3) 1 grams of potassium fluoride is dissolved to make 0 ml of. Molarity is represented by the following equation: We already have the molarity and volume of solution, both of which. Web calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. Ml of solution made from 0.70 moles of licl and water? What is the molarity of 500. Molarity is represented by the following equation: To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? 724.4 g of ammonium phosphate in 4500 ml of alcohol. This worksheet can be used in any chemistry class, regardless of the students' ability level. Web molarity and molality practice worksheet. 69.1 grams 2) how many liters of 4. We already have the molarity and volume of solution, both of which have good units. Moles of solute 4.00 m = 12.0 l moles of solute = 48.0 mol 2. What is the concentration of a solution of nacl if 40 ml of a 2 m nacl solution is diluted to a total volume of 500 ml? Show all work and circle your final answer. The molarity of the solution = 2 moles of solvent/1 liters of solution = 2 m solution. The students will need a periodic table to obtain molecular weights, if you choose to include mass instead of moles of solute. This worksheet can be used in any chemistry class, regardless of the students' ability level. Calculate grams ca (oh) 2 needed to completely neutralize 50.0 ml of 3.00 m. L = 250 ml × = 0.25 l 1000 ml moles of solute 0.150 m = 0.25 l 69 grams 2) how many liters of 4 m solution can be made using 100 grams of lithiumbromide? Web learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Molarity = moles of solute / volume of solution in litre. Molarity is abbreviated as m. How many moles of sucrose are dissolved in 250 ml of solution if the solution concentration is 0.150 m? To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? 69.1 grams 2) how many liters of 4 m solution can be made using 100 grams of lithium bromide? Let’s rewrite the equation to figure out how many moles there are. Ml of solution made from 0.70 moles of licl and water? 0.29 l 3) what is the concentration of an aqueous solution with a volume of 450 mlthat contains 200 grams of iron (ii) chloride? A quantitative description of solution concentration. Web calculate the molarity of each of the following solutions: Molarity = moles of solute / volume of solution in litre. Zn (no 3) 2 alcl 3 agbr fepo 4 cuac 2. Solutions to the molarity practice worksheet for the first five problems, you need to use the equation that says that the molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. We may do this by multiplying both sides of the equation by the l solution. 3.47 l 3) what is the concentration of an aqueous solution with a volume of 450 ml that contains 200 grams of iron (ii. Calculate grams of solute needed to prepare 225 ml of 0.400 m kbr solution. Web molarity practice worksheet find the molarity of the following solutions: Calculate ml of 0.650m kno 3 needed to contain 25.0g kno 3. Web this customizable and printable worksheet is designed to help students practice calculating the molarity of various solutions. Web the formula for calculating molarity when the moles of the solute and liters of the solution are given is = moles of solute/ liters of solution. Let’s rewrite the equation to figure out how many moles there are. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? What is the molarity of a solution containing 325 g of nacl dissolved in 750. Molarity answer key part 1 molarity:Molarity Worksheet Answers Chemistry Ivuyteq
️Molar Volume Worksheet Answer Key Free Download Goodimg.co
31 Molarity Worksheet Answer Key Education Template
Molarity Worksheet Answer Key
Molarity Practice Worksheet Answer
Molarity Worksheet 1 worksheet
7 Molarity Worksheet With Answers /
31 Molarity Worksheet Answer Key Education Template
Molarity, Molality, Normality, and Mass Percent Worksheet II Answer Key
️Molarity Practice Worksheet Free Download Goodimg.co
69.1 Grams 2) How Many Liters Of 4 M Solution Can Be Made Using 100 Grams Of Lithium Bromide?
Web Answer Key Included!Check Out All My Resources For Solutions:factors Affecting Solubility Labsolutions Vocab Slidessolubility Curve (Graphs) Notes & Slidessolution Vocabulary Dominossolubility Curves Graphing & Questionsgas Solubility Mini Labsolutions Review Stations Subjects:
What Is The Concentration Of A Solution Of Nacl If 40 Ml Of A 2 M Nacl Solution Is Diluted To A Total Volume Of 500 Ml?
Calculate Ml Of 0.650M Kno 3 Needed To Contain 25.0G Kno 3.
Related Post: