Molarity Practice Worksheet
Molarity Practice Worksheet - 2) 0.5 grams of sodium chloride is dissolved to make 0.05 liters of solution. 0.444 mol of cocl 2 in 0.654 l of solution 98.0 g of phosphoric acid, h 3 po 4, in 1.00 l of. The molarity of a mixture, mmix, can be calculated using the following formula: Web the molarity of a solution is measured in moles of solute per liter of solution, or mol/liter. Find the molarity of the following solutions: Moles of solute 4.00 m = 12.0 l moles of solute = 48.0 mol 2. Zero points will be given if. 6) if you take 1.0 liter of 2.0 molar nacl and dilute it to a volume of. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. This worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and. This worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. The correct option is ‘c’. What is the molarity of 500. Web molarity extra practice worksheet what is the molarity of 2.0 l of solution made. If molarity = then rearranging we find that moles of solute =. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. Calculate grams of solute needed to. 0.444 mol of cocl 2 in 0.654 l of solution 98.0 g of phosphoric acid, h 3 po 4, in 1.00 l. 1) in this problem, simply solve using the molarity equation to find that. Web molarity extra practice worksheet what is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Web molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Molarity = mole/liters. What is the molarity of 500. A complete answer key is provided at the end. Web calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Web molarity practice worksheet find the molarity of the following solutions: The molarity of a mixture, mmix, can be calculated using the following formula: 6) if you take 1.0 liter of 2.0 molar nacl and dilute it to a volume of. M mix = (m 1 v 1 + m 2 v 2) / (v 1 + v 2) = (1.5 x. What is the molarity of 500. Web answer problem 6.1.3 determine the molarity for each of the following solutions: 2) 0.5 grams. Calculate grams of solute needed to. What is the molarity of 500. Find the molarity of the following solutions: A complete answer key is provided at the end. How many liters of water are needed to make a 4.00 m solution. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Please show all steps for credit. Web molarity extra practice worksheet what is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? 1) 0.5 moles of sodium chloride is dissolved to make 0.05 liters of solution. This worksheet provides many examples for students. 0.444 mol of cocl 2 in 0.654 l of solution 98.0 g of phosphoric acid, h 3 po 4, in 1.00 l of. The correct option is ‘c’. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Find the molarity of the following solutions: For example, if the molarity of a mercury solution is 1m, it simply means. Web molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. M mix = (m 1 v 1 + m 2 v 2) / (v 1 + v 2) = (1.5 x. What is the molarity of 500.. The correct option is ‘c’. Calculate grams of solute needed to. This worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and. Moles of solute 4.00 m = 12.0 l moles of solute = 48.0 mol 2. Web molarity extra practice worksheet what is the molarity of 2.0 l of solution made from 2.4 moles of nacl. 0.444 mol of cocl 2 in 0.654 l of solution 98.0 g of phosphoric acid, h 3 po 4, in 1.00 l of. Find the molarity of the following solutions: Web molarity & dilutions practice problems. The correct option is ‘c’. For example, if the molarity of a mercury solution is 1m, it simply means that. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. This worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and. M mix = (m 1 v 1 + m 2 v 2) / (v 1 + v 2) = (1.5 x. Web molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. This worksheet provides many examples for students to practice calculations involving molarity. Web answer problem 6.1.3 determine the molarity for each of the following solutions: What is the molarity of a solution containing 4.20 moles of sulfuric acid in 300.0 ml of solution? Please show all steps for credit. How many liters of water are needed to make a 4.00 m solution. A complete answer key is provided at the end. Web molarity practice problems #1 grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? What is the molarity of a solution containing 325 g of nacl. If molarity = then rearranging we find that moles of solute =. 1) in this problem, simply solve using the molarity equation to find that. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Find the molarity of the following solutions: What is the molarity of a solution containing 325 g of nacl. Web molarity & dilutions practice problems. Web molarity practice problems #1 grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? 3) 0.5 grams of sodium chloride is dissolved to make 0.05 ml of solution. This worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and. Web molarity practice worksheet find the molarity of the following solutions: To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? If molarity = then rearranging we find that moles of solute =. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. Molarity = mole/liters (1 l = 1000 ml). M mix = (m 1 v 1 + m 2 v 2) / (v 1 + v 2) = (1.5 x. The correct option is ‘c’. Zero points will be given if. Please show all steps for credit. What is the resulting molarity?Molarity Practice Worksheet Answer
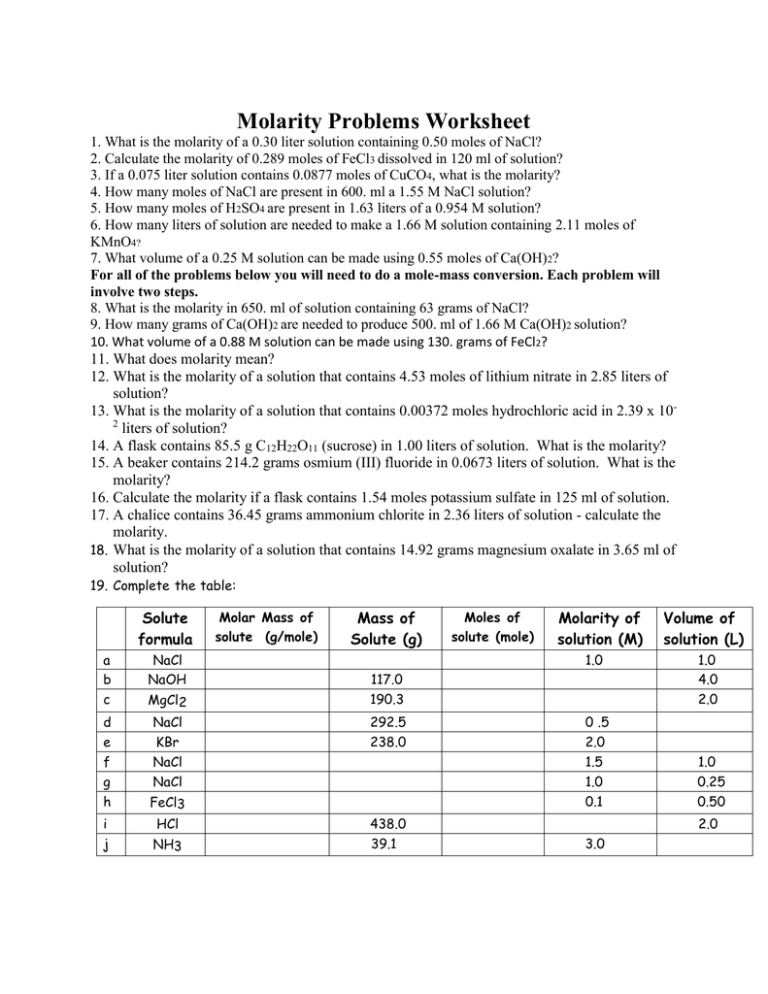
Molarity Problems Worksheet
Molarity Practice Problems Worksheet Answers
7 Molarity Worksheet With Answers /
Molarity Practice Worksheet
Molarity Practice Worksheet Answer
7 Molarity Worksheet With Answers /
️Molarity Problems Worksheet Free Download Gmbar.co
Molarity Practice Worksheets
Molarity Practice Worksheet Answer New Molarity Practice Worksheet
6) If You Take 1.0 Liter Of 2.0 Molar Nacl And Dilute It To A Volume Of.
This Worksheet Provides Many Examples For Students To Practice Calculations Involving Molarity.
The Molarity Of A Mixture, Mmix, Can Be Calculated Using The Following Formula:
0.444 Mol Of Cocl 2 In 0.654 L Of Solution 98.0 G Of Phosphoric Acid, H 3 Po 4, In 1.00 L Of.
Related Post: