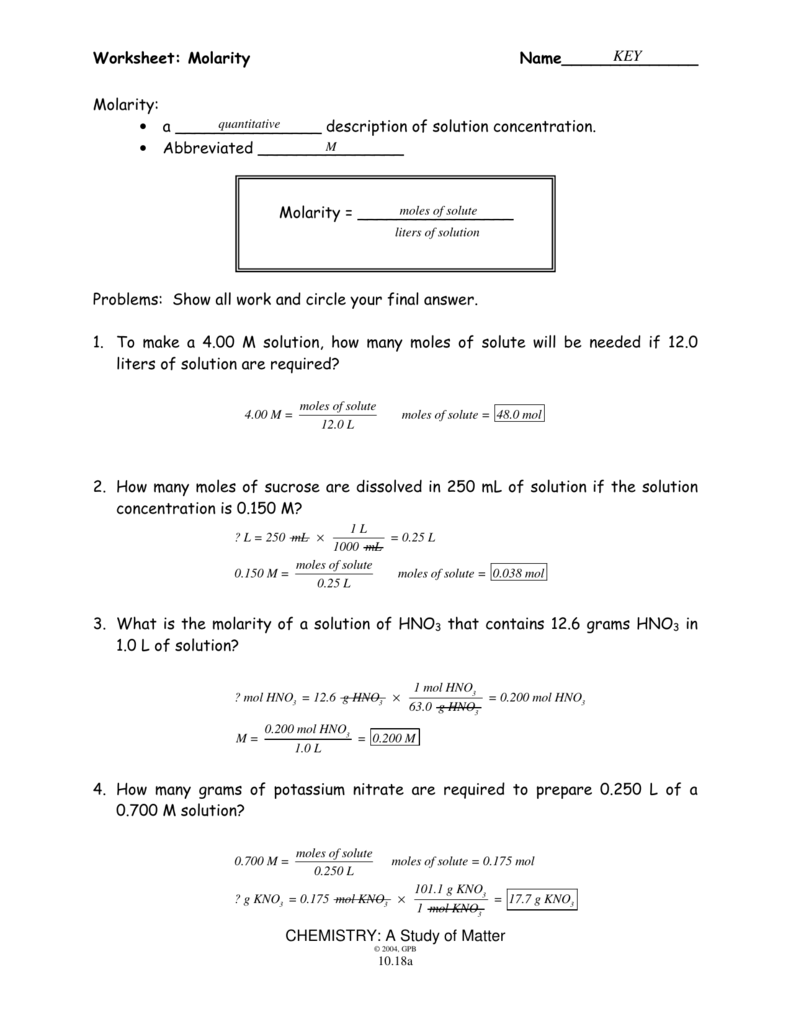
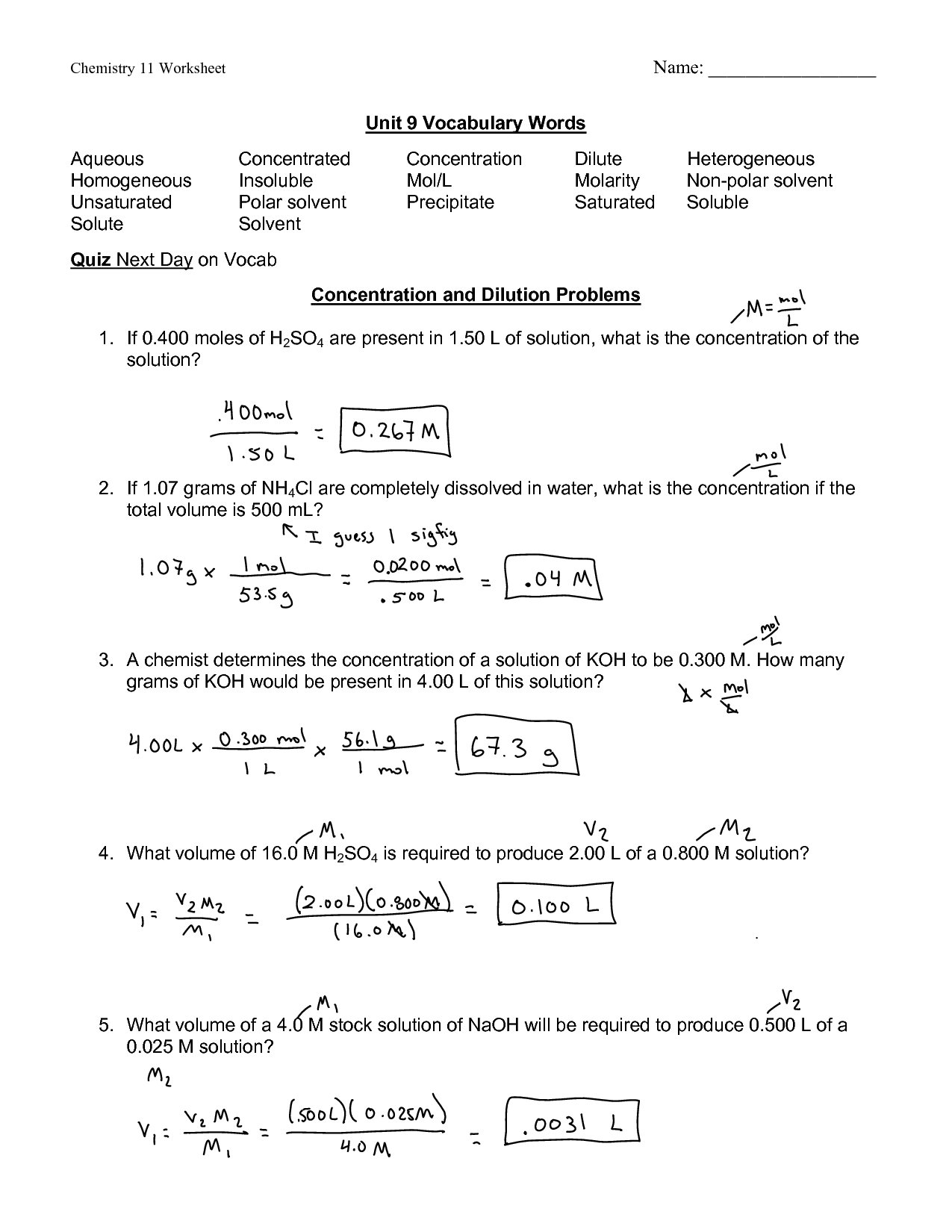
Molarity Problems Worksheet Answers
Molarity Problems Worksheet Answers - Calculate the molarity of each of the following solutions: Determine the molarity of these solutions: Such mixture is called a. Web a 2.5 m solution? Web best used as flashcards as a way to practice molarity type of problems. Web molarity practice worksheet find the molarity of the following solutions: Moles of solute 4.00 m = 12.0 l moles of solute = 48.0 mol 2. What is the molarity of a solution containing 325 g of nacl. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. 2.5 m solution the formula for calculating molarity when the moles of the solute and liters of the solution are given. Calculate the molarity of each of the following solutions: 0.5 grams of sodium chloride. 2) how many liters of 4 m solution can be made using 100 grams of lithium bromide? Moles of solute 4.00 m = 12.0 l moles of solute = 48.0 mol 2. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? Web solutions molarity worksheet name: Web solution the molarity is calculated by the following formula: 30 g of co (no 3) 2.6h 2 o in 4.3l of solution; 30 ml of 0.5m h 2 so 4 diluted to 500 ml. You have the option to present the amount of solutes to the students as moles, grams, or a mixture of. 30 ml of 0.5m h 2 so 4 diluted to 500 ml. 0.5 grams of sodium chloride. Determine the molarity of these solutions: Mv = grams / molar mass (x) (0.4000 l) = 5.30 g / 105.988 g mol¯1 0.12501415 m x = 0.125 m (to three sig figs) problem #4:what is the molarity of 5.00 g of naoh in.. You have the option to present the amount of solutes to the students as moles, grams, or a mixture of. Learn with flashcards, games, and more — for free. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. 3) what is the concentration of an aqueous solution with a. 30 ml of 0.5m h 2 so 4 diluted to 500 ml. Such mixture is called a. Web molarity practice worksheet find the molarity of the following solutions: Web the molarity worksheet maker generates up to 10 problems on each worksheet using a customized algorithm to produce problems that are realistic and unique. 2.5 m solution the formula for calculating. Web best used as flashcards as a way to practice molarity type of problems. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. 30 g of co (no 3) 2.6h 2 o in 4.3l of solution; M = n (mol)/v (l) first, determines the moles of sodium oxalate: What. A) x = 4.67 mol / 2.04 l b) x = 0.629 mol / 1.500 l c) (x) (10.00 l) = 4.783 g / 106.0 g mol¯1 d) (x) (0.250 l) = 0.897 g / 96.09 g. 3) what is the concentration of an aqueous solution with a volume of. 30 ml of 0.5m h 2 so 4 diluted to. A) x = 4.67 mol / 2.04 l b) x = 0.629 mol / 1.500 l c) (x) (10.00 l) = 4.783 g / 106.0 g mol¯1 d) (x) (0.250 l) = 0.897 g / 96.09 g. When we dissolve a cube of sugar in one cup of water, we create a homogeneous mixture. Moles of solute 4.00 m =. 30 g of co (no 3) 2.6h 2 o in 4.3l of solution; To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? Moles of solute 4.00 m = 12.0 l moles of solute = 48.0 mol 2. Web a 2.5 m solution? 4) 0.5 moles of sodium chloride. You have the option to present the amount of solutes to the students as moles, grams, or a mixture of. N (na 2 c 2 o 4) = 33.5 g / 134 g/mol = 0.25. When we dissolve a cube of sugar in one cup of water, we create a homogeneous mixture. 0.5 grams of sodium chloride. 2.5 m solution. Many aspects of the problems can be customized to best fit the needs of your students. Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions problems using m1v1=m2v2. Web a 2.5 m solution? Mv = grams / molar mass (x) (0.4000 l) = 5.30 g / 105.988 g mol¯1 0.12501415 m x = 0.125 m (to three sig figs) problem #4:what is the molarity of 5.00 g of naoh in. Calculate the molarity of each of the following solutions: What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? N (na 2 c 2 o 4) = 33.5 g / 134 g/mol = 0.25. Web solution the molarity is calculated by the following formula: Calculate the molarity of 0.289 moles of fecl3 dissolved in 120 ml of solution? Moles of solute 4.00 m = 12.0 l moles of solute = 48.0 mol 2. 2.5 m solution the formula for calculating molarity when the moles of the solute and liters of the solution are given. 3) what is the concentration of an aqueous solution with a volume of. When we dissolve a cube of sugar in one cup of water, we create a homogeneous mixture. What is the molarity of a solution containing 325 g of nacl. Such mixture is called a. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? You have the option to present the amount of solutes to the students as moles, grams, or a mixture of. Web molarity practice worksheet find the molarity of the following solutions: Web solutions molarity worksheet name: Determine the molarity of these solutions: 2.5 m solution the formula for calculating molarity when the moles of the solute and liters of the solution are given. 4) 0.5 moles of sodium chloride is dissolved to make 0.05 liters of solution. 0.5 grams of sodium chloride. Web this worksheet features 5 molarity problems (m=mol/l) with conversions from grams to moles and milliliters to liters and 7 dilutions problems using m1v1=m2v2. Many aspects of the problems can be customized to best fit the needs of your students. What is the molarity of a solution containing 325 g of nacl. Determine the molarity of these solutions: 3) what is the concentration of an aqueous solution with a volume of. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are required? Such mixture is called a. Mv = grams / molar mass (x) (0.4000 l) = 5.30 g / 105.988 g mol¯1 0.12501415 m x = 0.125 m (to three sig figs) problem #4:what is the molarity of 5.00 g of naoh in. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. 30 ml of 0.5m h 2 so 4 diluted to 500 ml. 30 g of co (no 3) 2.6h 2 o in 4.3l of solution; Web molarity practice worksheet find the molarity of the following solutions: A) x = 4.67 mol / 2.04 l b) x = 0.629 mol / 1.500 l c) (x) (10.00 l) = 4.783 g / 106.0 g mol¯1 d) (x) (0.250 l) = 0.897 g / 96.09 g.Molarity Worksheet Answer Key
Molarity Practice Worksheet Answer
14 Chemistry Mole Practice Worksheet /
Molarity Practice MS MCLARTY'S CLASSES
7 Molarity Worksheet With Answers /
31 Molarity Worksheet Answer Key Education Template
Molarity Practice Worksheet Answer
Molarity Worksheet 1 worksheet
Molarity Worksheet 1 Answer Key Chemistry
7 Molarity Worksheet With Answers /
You Have The Option To Present The Amount Of Solutes To The Students As Moles, Grams, Or A Mixture Of.
Web Solution The Molarity Is Calculated By The Following Formula:
What Is The Molarity Of A 0.30 Liter Solution Containing 0.50 Moles Of Nacl?
Calculate The Molarity Of Each Of The Following Solutions:
Related Post: