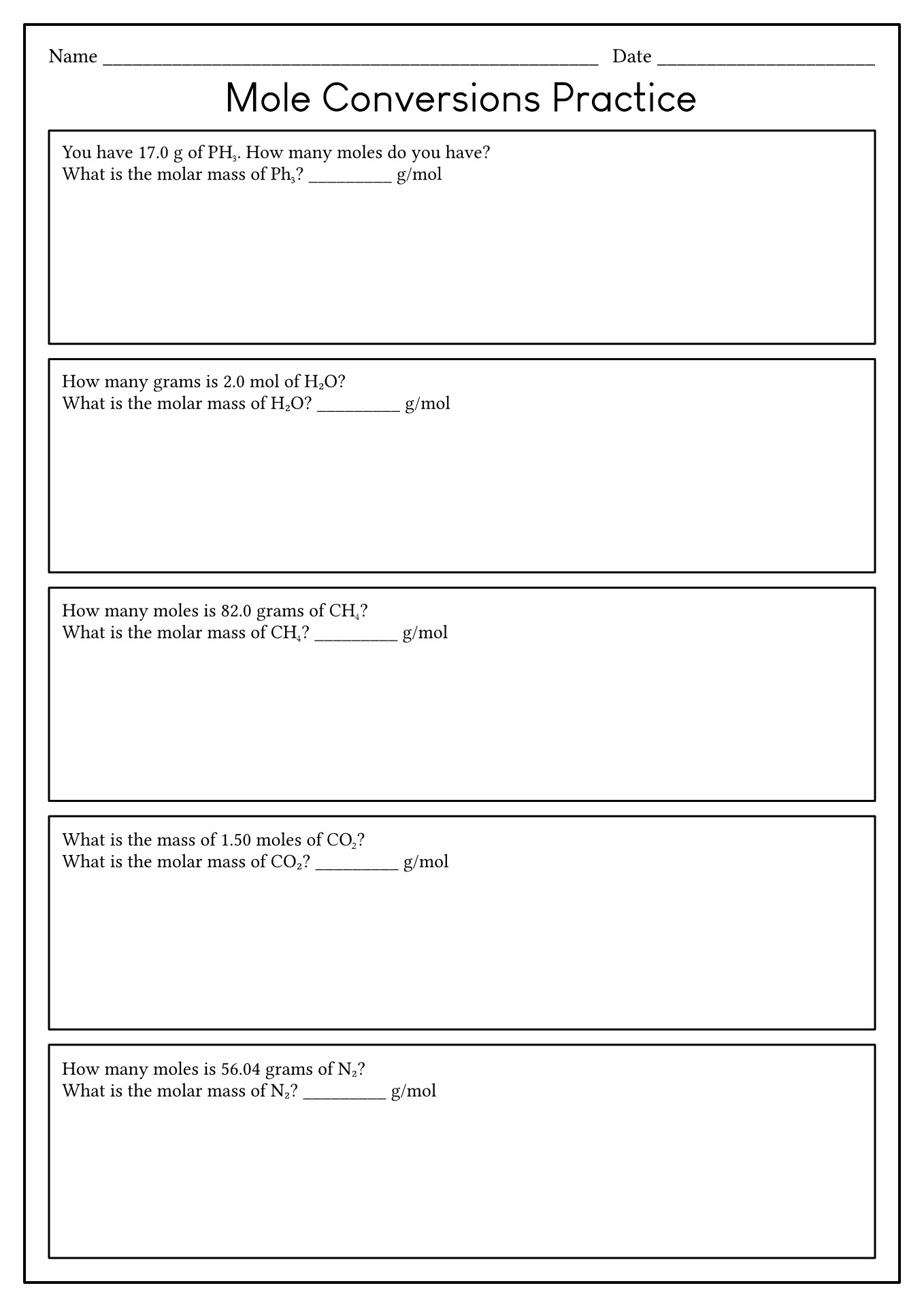
Mole Conversion Worksheet Answers
Mole Conversion Worksheet Answers - 25 g nacl 25g nacl x 1mol nacl 58.5g nacl= 0.43molnacl b. Determine how many moles of gold atoms you have. You should try to answer the questions without referring to your textbook. Expert level (hint you must use. 23 2.43 mol ag atoms 1.46 10 ag atoms6.022 10 ag atoms 24 1 mol ag atoms ×. How many moles of magnesium are in 3.01 x 1022 atoms of magnesium? 0.160 mol h2o 2.88 g g. 1 mole = 6.02 x 1023 particles 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp (you do not need to worry about this yet) The mass of 11 mol of hydrogen chloride. How many ag atoms are in 2.43 mol ag atoms? The mass of 11 mol of hydrogen chloride. 1 mole = 6.02 x 1023 particles 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp (you do not need to worry about this yet) 23 2.43 mol ag atoms 1.46 10 ag atoms6.022 10 ag. Web this is an answer key for the worksheet mole conversion practice. Web the number of moles of 54.9 grams in k 2. The number of moles of 99.4 grams of nacl. Molar is obtained from moles, and therefore, the molar mass is the mass of a substance in grams, in one mole of that particular substance. 23 2.43 mol. Expert level (hint you must use. Web all about moles and how to convert them into different units e1 5g mole conversions worksheet there are three mole equalities. Web mole conversions worksheet there are three mole equalities. How many moles are 1.20. Web using the factor label method: Web study with quizlet and memorize flashcards containing terms like how many moles of magnesium is 3.01 x 10^22 atoms of magnesium?, how many molecules are there in. Web 1) naoh 2) h3po4 3) h2o 4) mn2se7 5) mgcl2 6) (nh4)2so4 there are three definitions (equalities) of mole. Show all work utilizing dimensional analysis wherever possible. Include all units and. 23 2.43 mol ag atoms 1.46 10 ag atoms6.022 10 ag atoms 24 1 mol ag atoms ×. How many moles are 1.20. Determine how many moles of gold atoms you have. Find the amount of moles contained in each specimen. Use a separate piece of paper, show all work, and circle your final answer. Expert level (hint you must use. 7.87 kg h 2 o 2 2.34 kg nacl 12.5 g c 2 h 6 o 85.72 g nh 3 strategy a. How many moles are in a. Determine how many moles of gold atoms you have. Use a separate piece of paper, show all work, and circle your final answer. Show all work utilizing dimensional analysis wherever possible. Web mole conversions worksheet there are three mole equalities. The number of moles of 99.4 grams of nacl. You weigh a 24 k gold chain, and find that it weighs 400 grams. Web the number of moles of 54.9 grams in k 2. You weigh a 24 k gold chain, and find that it weighs 400 grams. Web mole conversions worksheet #1 1. Include all units and account for significant figures. Determine how many moles of gold atoms you have. 1 mole = 6.02 x 1023 particles 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1. Web this is an answer key for the worksheet mole conversion practice. Web mole conversions and stoichiometry worksheet 1. 120 g kmno4 120g kmno4x 1mol kmno4 158.0g kmno4=0.76mol kmno4 c. Find the amount of moles contained in each specimen. Expert level (hint you must use. Include all units and account for significant figures. Web the number of moles of 54.9 grams in k 2. Web work in groups on these problems. 120 g kmno4 120g kmno4x 1mol kmno4 158.0g kmno4=0.76mol kmno4 c. Show all work utilizing dimensional analysis wherever possible. Web 1) naoh 2) h3po4 3) h2o 4) mn2se7 5) mgcl2 6) (nh4)2so4 there are three definitions (equalities) of mole. 7.87 kg h 2 o 2 2.34 kg nacl 12.5 g c 2 h 6 o 85.72 g nh 3 strategy a. Use a separate piece of paper, show all work, and circle your final answer. How many ag atoms are in 2.43 mol ag atoms? There are some examples of how to complete the equations and mole conversion throughout the study. Web work in groups on these problems. The mass of 11 mol of hydrogen chloride. Web all about moles and how to convert them into different units e1 5g mole conversions worksheet there are three mole equalities. Expert level (hint you must use. Web mole conversions worksheet there are three mole equalities. The number of moles of 99.4 grams of nacl. 1 mole = 6.02 x 1023 particles 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp (you do not need to worry about this yet) You weigh a 24 k gold chain, and find that it weighs 400 grams. How many moles of magnesium are in 3.01 x 1022 atoms of magnesium? Web the number of moles of 54.9 grams in k 2. Molar is obtained from moles, and therefore, the molar mass is the mass of a substance in grams, in one mole of that particular substance. 120 g kmno4 120g kmno4x 1mol kmno4 158.0g kmno4=0.76mol kmno4 c. Web up to 24% cash back name period mole worksheet #2 make the following conversions using unit analysis. 23 2.43 mol ag atoms 1.46 10 ag atoms6.022 10 ag atoms 24 1 mol ag atoms ×. Determine how many moles of gold atoms you have. Web mole conversions worksheet there are three mole equalities. Expert level (hint you must use. Molar is obtained from moles, and therefore, the molar mass is the mass of a substance in grams, in one mole of that particular substance. 0.160 mol h2o 2.88 g g. Web mole conversions worksheet #1 1. Web the number of moles of 54.9 grams in k 2. Web study with quizlet and memorize flashcards containing terms like how many moles of magnesium is 3.01 x 10^22 atoms of magnesium?, how many molecules are there in. Web mole to mole conversionto teach students how to convert from mole to mole in stoichiometry. How many moles are in a. How many molecules are there in 4.00 moles of glucose, c6h12o6? You should try to answer the questions without referring to your textbook. How many moles of magnesium are in 3.01 x 1022 atoms of magnesium? Web this is an answer key for the worksheet mole conversion practice. Web mole conversion worksheet mole conversion worksheetname:______________________________ there are three mole equalities. If you get stuck, try asking another group for help. 1 mole = 6.02 x 1023 particles 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp (you do not need to worry about this yet)14 Chemistry Mole Practice Worksheet /
Mole Conversion Worksheet With Answers Printable Math —
11 Moles And Mass Worksheet Answers /
10 Best Images of Moles And Mass Worksheet Answers Moles and Molar
stoichiometry worksheet mole to mole answers
18 Mass And Moles Worksheet Answer Key /
Mole To Grams Grams To Moles Conversions Worksheet Answer Key — db
List Of Worksheet Mole Mole Problems 2023 Hugh Worksheet
18 Best Images of Mole Conversion Problems Worksheet Answers Mole Ratio
️Mole Conversion Worksheet Answers Free Download Gambr.co
Web Mole Conversions And Stoichiometry Worksheet 1.
Web All About Moles And How To Convert Them Into Different Units E1 5G Mole Conversions Worksheet There Are Three Mole Equalities.
Determine How Many Moles Of Gold Atoms You Have.
Web Using The Factor Label Method:
Related Post: