Molecular Geometry Worksheet Answers
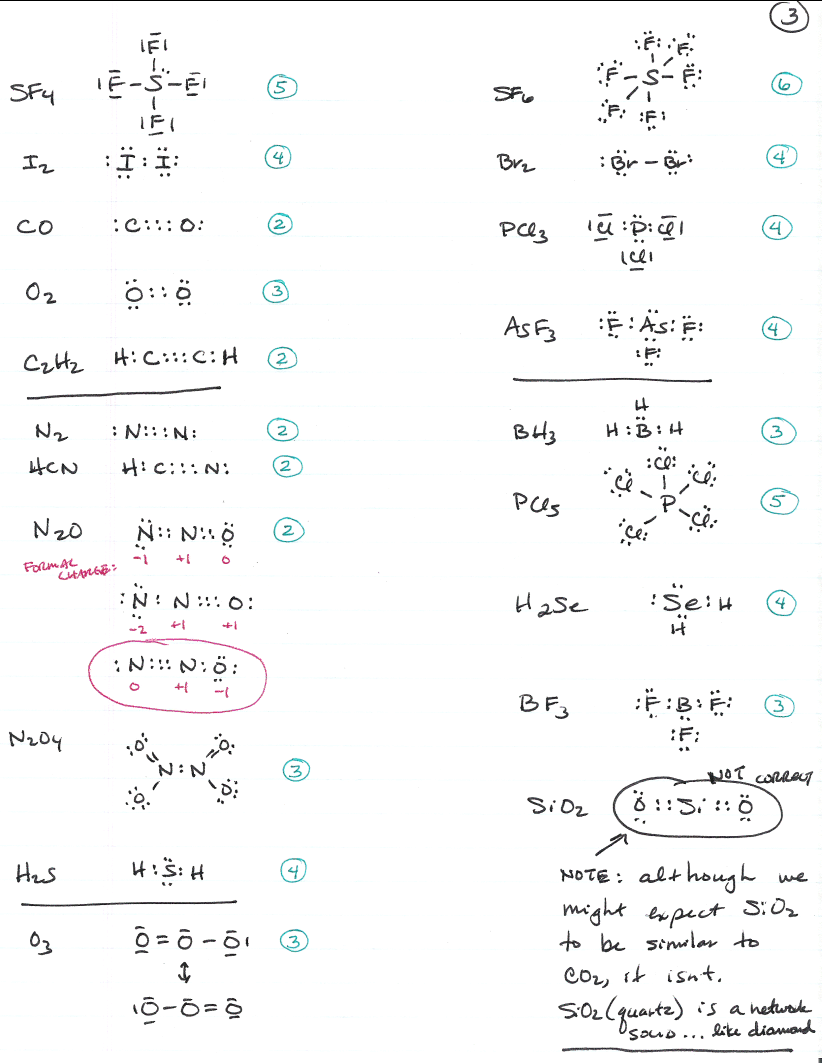
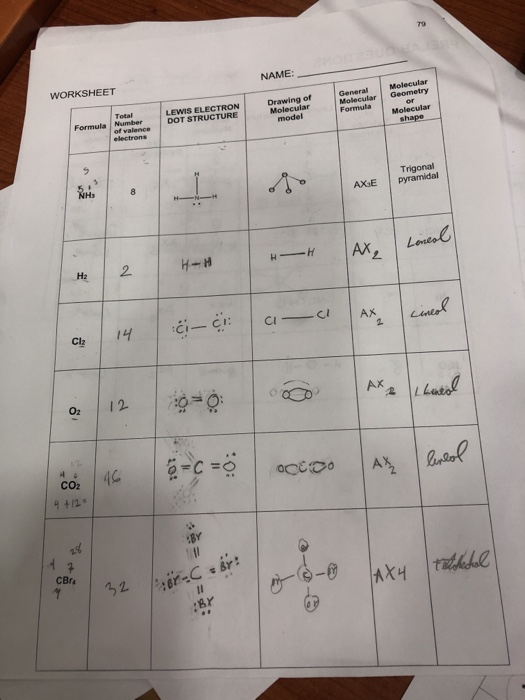
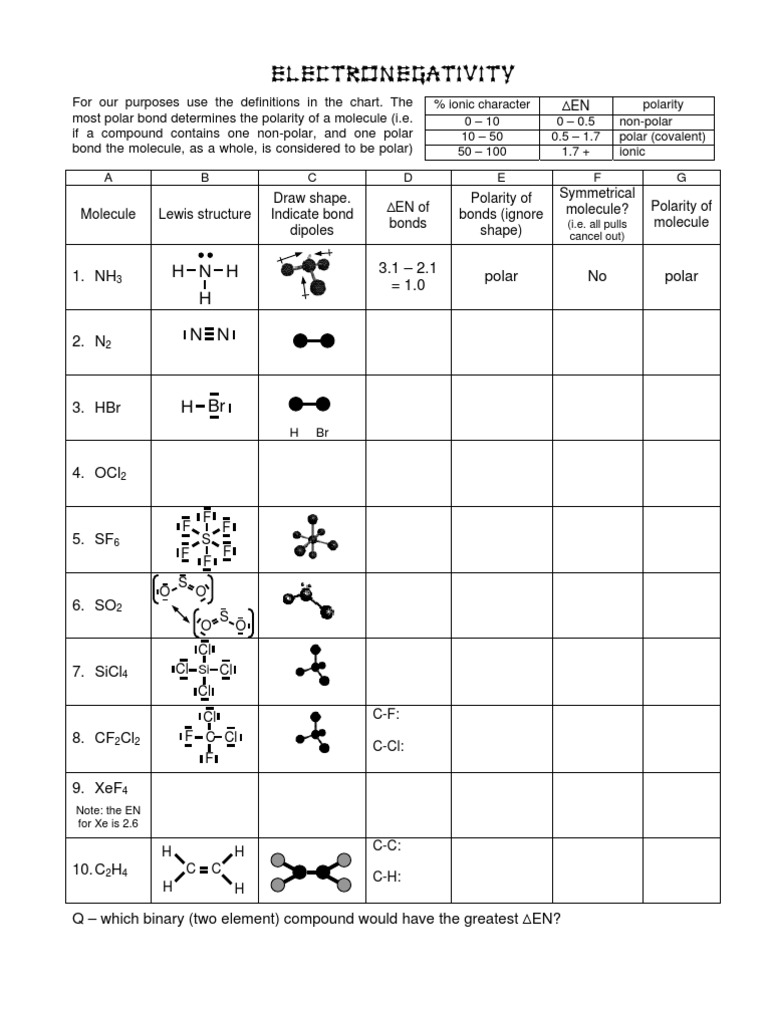
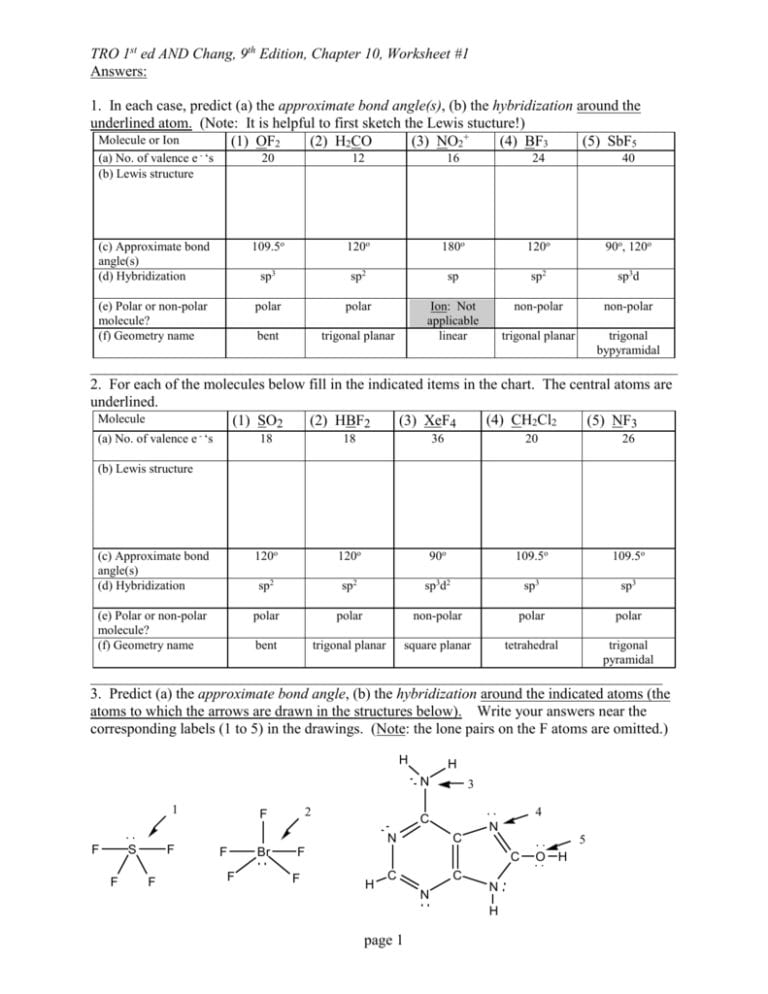
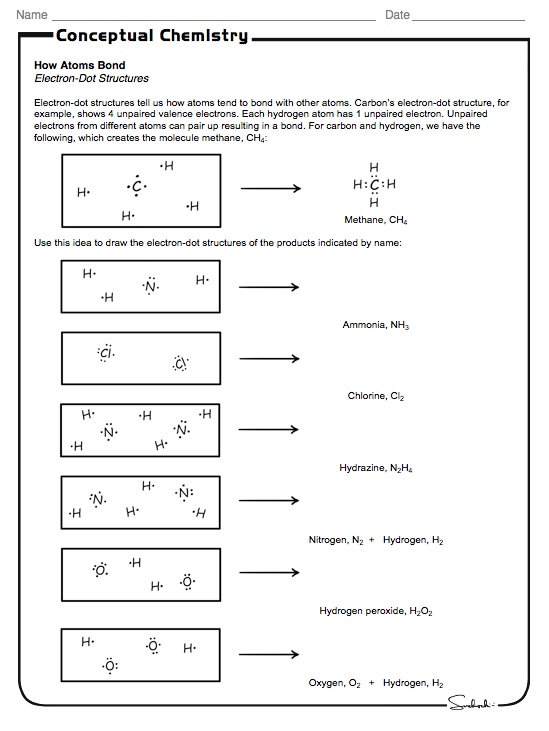
Molecular Geometry Worksheet Answers - A) draw the lewis (electron dot) structures of pf3 and pf4+ and use the vsepr theory to. Page 6 lewis structure classification electron pair geometry (epg) molecular geometry (mg) bond angle(s. Web up to 24% cash back identifying molecular geometries. Episode 501” or book p. Web download molecular geometry worksheet answer key and more chemistry exercises in pdf only on docsity! Best lewis structure electron geometry molecular geometry. Some of the worksheets for this concept are 5 1920 molecular geometry and forces wkst,. In each case, predict (a) the approximate bond angle(s) , (b) the hybridization around the underlined atom. The number of bonding pairs in a. Web explore molecule shapes by building molecules in 3d! Draw the lewis structure including any resonance structures if they apply. Some of the worksheets for this concept are 5 1920 molecular geometry and forces wkst,. (refer to “note taking guide: The number of bonding pairs in a. It is helpful to first sketch the lewis stucture!) molecule or ion Web download molecular geometry worksheet answer key and more chemistry exercises in pdf only on docsity! Mel forte how can molecular shapes be pi icted using the. Applying valence bond theory to the hybridization of atomic orbitals. This resource is a helpful guide to walk students through the phet simulation for molecular geometry. How does molecule shape change with different. Web chapter 8 lewis structures, electron & molecular geometry worksheet #2. In molecules, hydrogen is always a terminal atom. It will give them step by step instructions on which bond type and. Web chemistry questions and answers. Web if they are oriented 180 o from each other their repulsions will be minimized. Based on the information in the lesson, which of. Web chapter 8 lewis structures, electron & molecular geometry worksheet #2. How does molecule shape change with different numbers of bonds and electron pairs? Web view molecular geometry worksheet answers.pdf from science 1039 at somerville high, somerville. The molecular geometry of a clf 3 molecule is best described as: In molecules, hydrogen is always a terminal atom. Web view molecular geometry worksheet answers.pdf from science 1039 at somerville high, somerville. Web download molecular geometry worksheet answer key and more chemistry exercises in pdf only on docsity! Find out by adding single, double or. Web if they are oriented 180 o from each other their repulsions will be minimized. It will give them step by step instructions on which bond type and. Web chemistry questions and answers. Page 6 lewis structure classification electron pair geometry (epg) molecular geometry (mg) bond angle(s. Best lewis structure electron geometry molecular geometry. Mel forte how can molecular shapes be pi icted using the. This resource is a helpful guide to walk students through the phet simulation for molecular geometry. In the first column of the chart on the next 3 pages, list if the bond is ionic, polar covalent or nonpolar covalent. Hybrid orbitals anonymous libretexts table of contents learning objectives the vsepr model note the pattern two electron groups ax2:. Best lewis. Distinguishing between electronic and molecular geometries. A) draw the lewis (electron dot) structures of pf3 and pf4+ and use the vsepr theory to. Draw the lewis structure including any resonance structures if they apply. Web chemistry questions and answers. How does molecule shape change with different numbers of bonds and electron pairs? Mel forte how can molecular shapes be pi icted using the. Hybrid orbitals anonymous libretexts table of contents learning objectives the vsepr model note the pattern two electron groups ax2:. The molecular geometry of a clf 3 molecule is best described as: It is helpful to first sketch the lewis stucture!) molecule or ion Page 6 lewis structure classification electron. The central atom in a molecules is the one with the highest electronegativity. It will give them step by step instructions on which bond type and. Web up to 24% cash back identifying molecular geometries. Web if they are oriented 180 o from each other their repulsions will be minimized. Mel forte how can molecular shapes be pi icted using. Therefore, the best electron pair geometry for two pairs is a linear arrangement. Page 6 lewis structure classification electron pair geometry (epg) molecular geometry (mg) bond angle(s. Mel forte how can molecular shapes be pi icted using the. Web chemistry questions and answers. Based on the information in the lesson, which of. Hybrid orbitals anonymous libretexts table of contents learning objectives the vsepr model note the pattern two electron groups ax2:. Best lewis structure electron geometry molecular geometry. (refer to “note taking guide: Web chapter 8 lewis structures, electron & molecular geometry worksheet #2. Web download molecular geometry worksheet answer key and more chemistry exercises in pdf only on docsity! Web view molecular geometry worksheet answers.pdf from science 1039 at somerville high, somerville. This resource is a helpful guide to walk students through the phet simulation for molecular geometry. Some of the worksheets for this concept are 5 1920 molecular geometry and forces wkst,. The molecular geometry of a clf 3 molecule is best described as: Draw the lewis structure including any resonance structures if they apply. Web if they are oriented 180 o from each other their repulsions will be minimized. In each case, predict (a) the approximate bond angle(s) , (b) the hybridization around the underlined atom. The number of bonding pairs in a. In molecules, hydrogen is always a terminal atom. Web targeted skills worksheet question 2 lewis (electron dot) structures are useful models. Page 6 lewis structure classification electron pair geometry (epg) molecular geometry (mg) bond angle(s. Web chapter 8 lewis structures, electron & molecular geometry worksheet #2. It is helpful to first sketch the lewis stucture!) molecule or ion Web chemistry questions and answers. A) draw the lewis (electron dot) structures of pf3 and pf4+ and use the vsepr theory to. Based on the information in the lesson, which of. Some of the worksheets for this concept are 5 1920 molecular geometry and forces wkst,. Episode 501” or book p. The central atom in a molecules is the one with the highest electronegativity. Distinguishing between electronic and molecular geometries. The number of bonding pairs in a. Web explore molecule shapes by building molecules in 3d! (refer to “note taking guide: Therefore, the best electron pair geometry for two pairs is a linear arrangement. In molecules, hydrogen is always a terminal atom. How does molecule shape change with different numbers of bonds and electron pairs?Molecule Shapes Answer Key › Athens Mutual Student Corner
41+ Molecular Geometry Model 1 Lewis Structures Answers PNG GM
Top Molecular Geometry Chart Answers Most Popular GM
Download Molecular Geometry Practice Sheet Answers Pics GrAffiTi
42+ Molecular Geometry Practice Pictures GrAffiTi
29+ Molecular Geometry Lab Answer Key Pics GM
Pogil Intermolecular Forces Worksheet Answer Key PSLK Best Answer Key
Get Hbr Molecular Geometry Shape Images GrAffiTi
Molecular Geometry Worksheet Answers —
Molecularshapeworksheetwithanswers tabellb
Find Out By Adding Single, Double Or.
Web Targeted Skills Worksheet Question 2 Lewis (Electron Dot) Structures Are Useful Models.
Draw The Lewis Structure Including Any Resonance Structures If They Apply.
Web View Molecular Geometry Worksheet Answers.pdf From Science 1039 At Somerville High, Somerville.
Related Post: