Moles To Particles Worksheet Answers
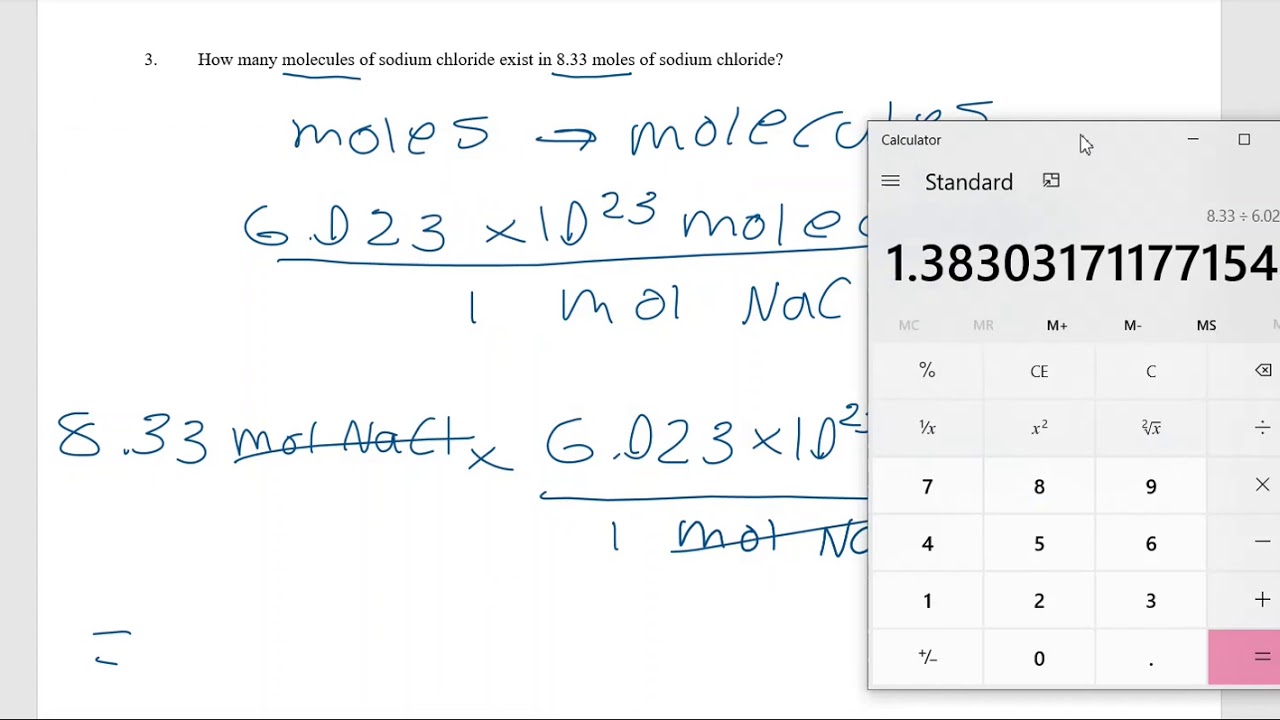
Moles To Particles Worksheet Answers - 3) how many grams are there. Define atomic mass, formula mass and molecular mass. You should try to answer the questions without referring to your textbook. Web one important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. 40.08 grams of calcium is one mole (see periodic table), and one mole is 6.02 x 1023 atoms. Web use this moles worksheet to show your students how to calculate the number of particles in a substance when given either the number of moles or the mass. How many formula units are there in 24 g of fef3? Web use this moles worksheet to show your students how to calculate the number of particles in a substance when given either the number of moles or the mass. Web moles to particles. 4) how many moles are in 3.4. Web use this moles worksheet to show your students how to calculate the number of particles in a substance when given either the number of moles or the mass. This worksheet has students answer simplified conversion questions (no word problems) to show understanding of the mole to particle conversion factor. Web use this moles worksheet to show your students how. 2) how many grams are in 4.5 moles of li 2 o? Define atomic mass, formula mass and molecular mass. Moles to particles (atoms or molecules) worksheet. 3 + na3po4→ alpo4 + 3nacl 44 g alcl358 g nacl. Web how many moles are 1.20 x 10^25 atoms of phosphorous? 3) how many grams are there. 5.47 x 1023 atoms ag. Moles to particles (atoms or molecules) worksheet. Web moles, mass and particles worksheet. Web 1) how many moles are in 25 grams of water? Web more than trillion exercisesclass copy. How many molecules are in 2.00 moles of h. Web how many moles are 1.20 x 10^25 atoms of phosphorous? Moles to particles (atoms or molecules) worksheet. Converting representative particles to moles: Web application this moles working to show your students how to calculate the number of particles in a substance when given either the quantity of moles or the mass. How many molecules are in 2.00 moles of h. This worksheet has students answer simplified conversion questions (no word problems) to show understanding of the mole to particle conversion factor. You. 3 + na3po4→ alpo4 + 3nacl 44 g alcl358 g nacl. Web 1) how many moles are in 25 grams of water? 4) how many moles are in 3.4. 6.02 x 1023 atoms ag. 3) how many grams are there. Web use this moles worksheet to show your students how to calculate the number of particles in a substance when given either the number of moles or the mass. 6.02 x 1023 atoms ag. 3) how many molecules are in 23 moles of oxygen? 5.47 x 1023 atoms ag. 4) how many moles are in 3.4. Web moles to particles. Web one important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. 3 + na3po4→ alpo4 + 3nacl 44 g alcl358 g nacl. Web use this moles worksheet to show your students how to calculate the number of particles in a substance when. 2) how many grams are in 4.5 moles of li 2 o? One mole of anything is 6.02 x 10 23, so it is 6.02 x 10 mg atoms. Web use this moles worksheet to show your students how to calculate the number of particles in a substance when given either the number of moles or the mass. Web define. How many formula units are there in 24 g of fef3? Web application this moles working to show your students how to calculate the number of particles in a substance when given either the quantity of moles or the mass. Define atomic mass, formula mass and molecular mass. 3 + na3po4→ alpo4 + 3nacl 44 g alcl358 g nacl. 4). 3) how many grams are there. While a dozen is only 12 particles a mole is a. One mole of anything is 6.02 x 10 23, so it is 6.02 x 10 mg atoms. 19.969 moles or 20.0 moles. Web one important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. Web define one mole of a substance as 6.02 x 1023 particles. 6.02 x 1023 atoms ag. Web used this moles worksheet to show your students wherewith to calculate this number of particles int a substance when given either of number of common or the mass. Web more than trillion exercisesclass copy. Web work in groups on these problems. If you get stuck, try asking another group for help. Web how many moles are 1.20 x 10^25 atoms of phosphorous? 2) how many formula units are there in 450 g of na2so4? How many formula units are there in 24 g of fef3? You should try to answer the questions without referring to your textbook. Web moles, mass and particles worksheet. Web use this moles worksheet to show your students how to calculate the number of particles in a substance when given either the number of moles or the mass. Web a mole of substance is that amount that contains as many elementary units (atoms, molecules, or formula units, depending on the nature of the substance) as there. Example 1) how many atoms are in 0.909 moles of silver? 3 + na3po4→ alpo4 + 3nacl 44 g alcl358 g nacl. 19.969 moles or 20.0 moles. If you get stuck, try asking another group for help. 6.02 x 1023 atoms ag. Web one important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. Web moles to particles. How many molecules are in 2.00 moles of h. Example 1) how many atoms are in 0.909 moles of silver? Moles to particles (atoms or molecules) worksheet. Web how many moles are 1.20 x 10^25 atoms of phosphorous? Converting representative particles to moles: You should try to answer the questions without referring to your textbook. Web application this moles working to show your students how to calculate the number of particles in a substance when given either the quantity of moles or the mass. Web use this moles worksheet to show your students how to calculate the number of particles in a substance when given either the number of moles or the mass. 2) how many formula units are there in 450 g of na2so4? Web work in groups on these problems. Web a mole of substance is that amount that contains as many elementary units (atoms, molecules, or formula units, depending on the nature of the substance) as there.Mole Conversion Worksheet Working With Moles And Particles Answer Key
Mole Conversions Worksheet 1
Chemistry Conversion Factors Worksheet
Moles to Particles Worksheet Solutions YouTube
14 Chemistry Mole Practice Worksheet /
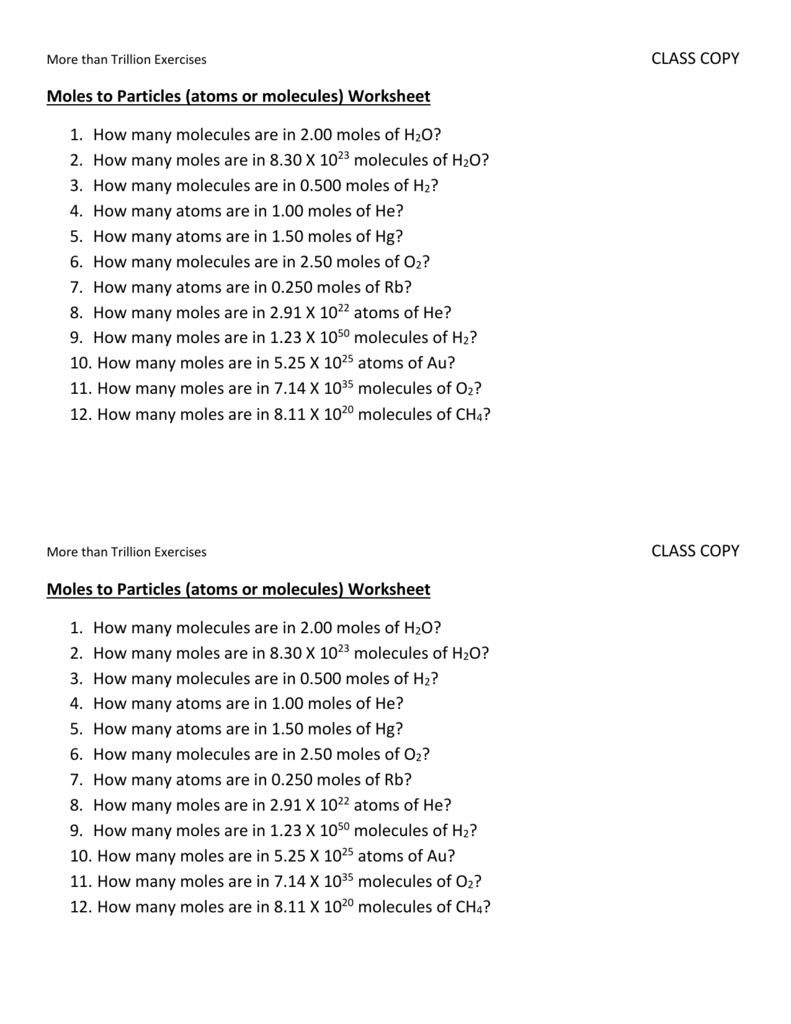
Moles to Particles (atoms or molecules) Worksheet
Moles To Particles Atoms Or Molecules Worksheet
10 Best Images of Moles And Mass Worksheet Answers Moles and Molar
Molarity Practice Worksheet Answer
Mole Conversions Worksheet Answer Key Chem 1A Studocu —
Web Used This Moles Worksheet To Show Your Students Wherewith To Calculate This Number Of Particles Int A Substance When Given Either Of Number Of Common Or The Mass.
This Worksheet Has Students Answer Simplified Conversion Questions (No Word Problems) To Show Understanding Of The Mole To Particle Conversion Factor.
5.47 X 1023 Atoms Ag.
Web More Than Trillion Exercisesclass Copy.
Related Post: