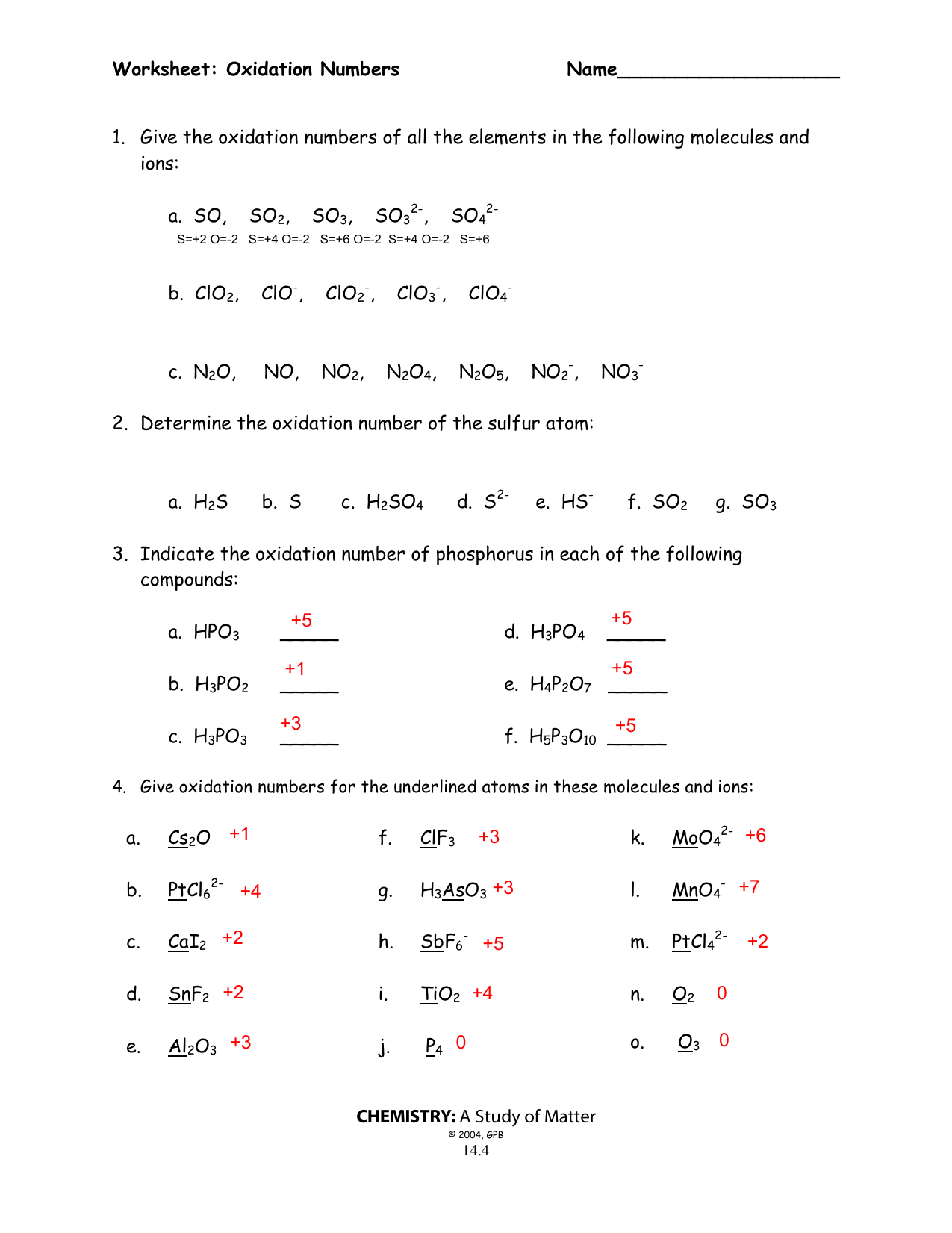
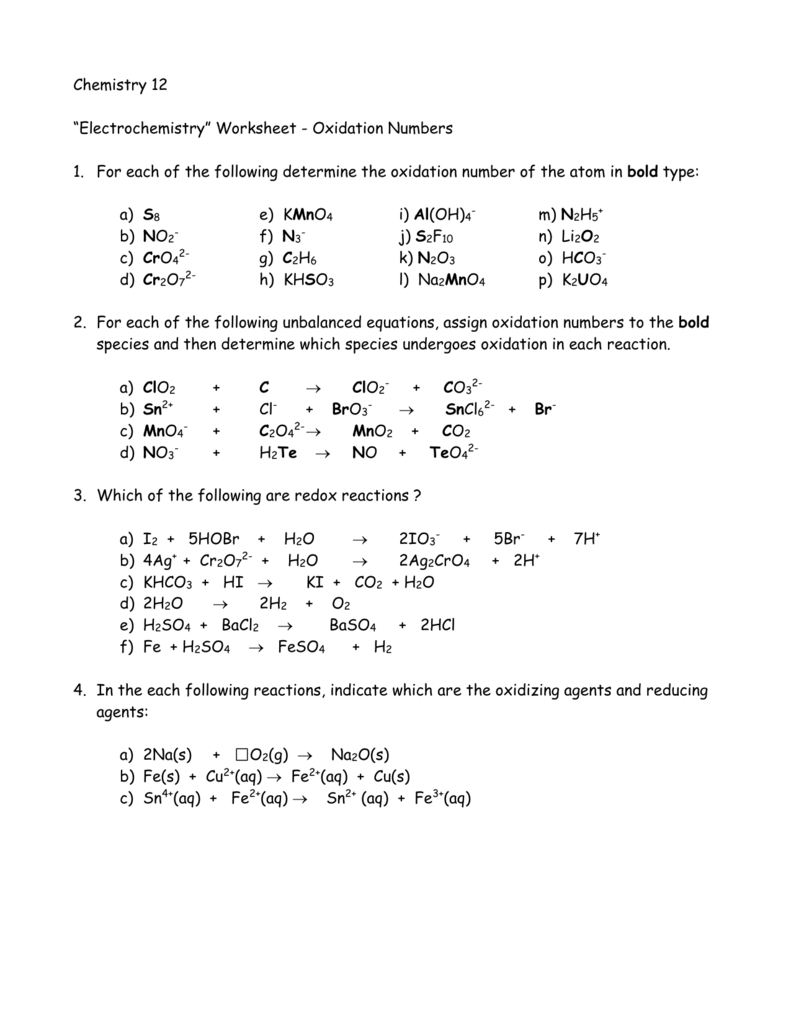
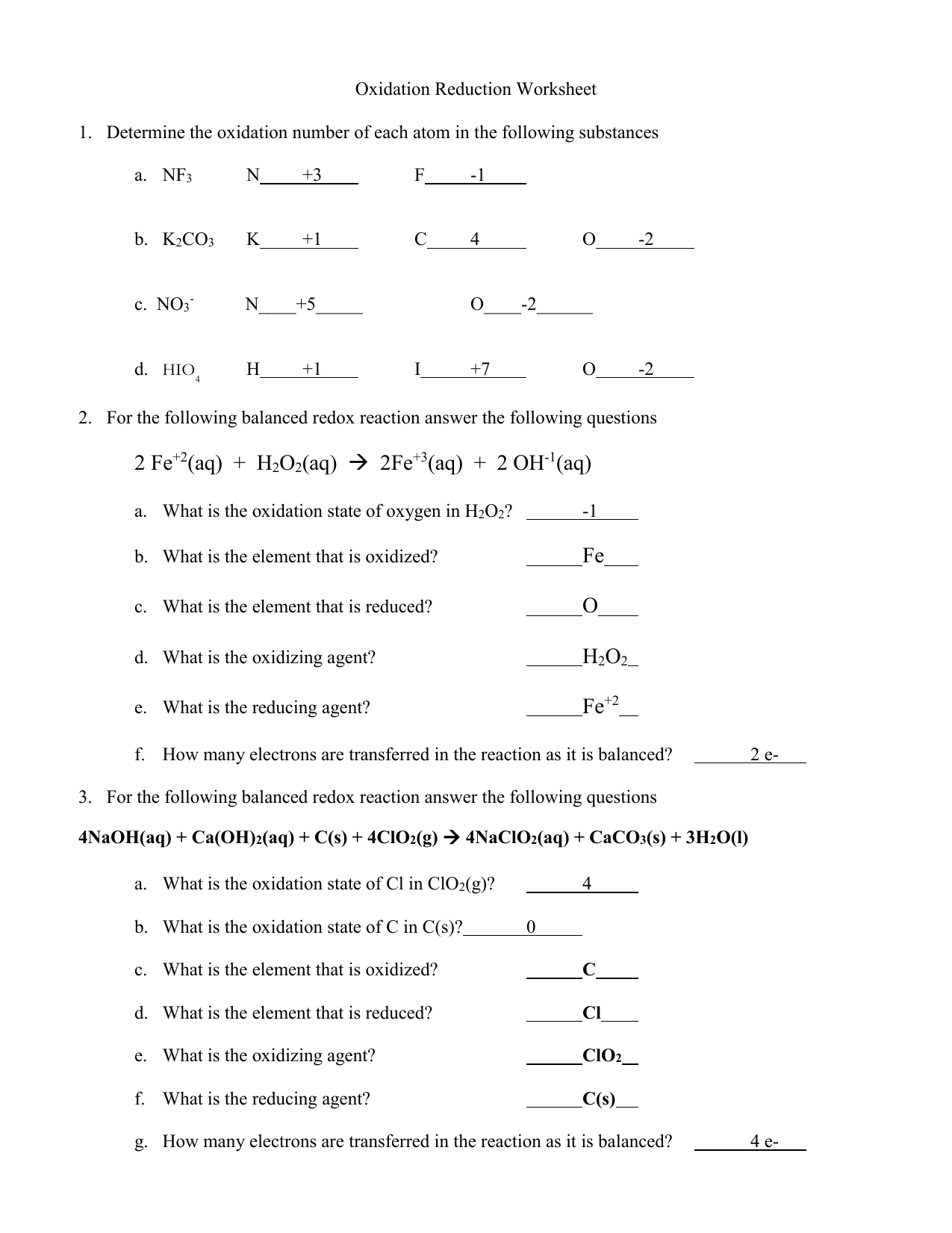
Oxidation Numbers Worksheet Answer Key
Oxidation Numbers Worksheet Answer Key - A pure element has an oxidation number of 0. The oxidation number of a monatomic ion equals the charge on the ion. The oxidation number of any pure element is 0. Web this product contains two pages. The handout outlines and illustrates the rules for assigning oxidation numbers. The more electronegative clement in a binary compound is assigned the number oqual to the charge it would have if it were an ion. The charge on all metals of group 2 of the periodic table is +2 d. Web rules for assigning oxidation numbers 1. Web $1.00 pdf this product contains two pages. Web identify the oxidation state of each element in the following: Web give the oxidation numbers of all the elements in the following molecules and ions: The oxidation number of a monatomic ion equals the charge on the ion. The worksheet gives students 10 compounds in which they must. In the following questions, give the oxidation. A pure element has an oxidation number of 0. Web $1.00 pdf this product contains two pages. The oxidation number of any pure element is 0. The handout outlines and illustrates the rules for assigning oxidation numbers. The worksheet gives students 10 compounds in which they must. Web give the oxidation numbers of all the elements in the following molecules and ions: The more electronegative clement in a binary compound is assigned the number oqual to the charge it would have if it were an ion. The charge on all free elements is zero. The charge on all metals of group 1 of the periodic table is +1 c. The oxidation number of a monatomic ion equals the charge on the ion.. The charge on all metals of group 1 of the periodic table is +1 c. The oxidation number of any pure element is 0. Web 1 the oxidation number of an element is zero. 2 for a monatomic ion, the oxidation number is the charge on the ion. Identify the oxidation state of nitrogen in the following: Web this product contains two pages. Web identify the oxidation state of each element in the following: A pure element has an oxidation number of 0. The charge on all metals of group 2 of the periodic table is +2 d. The charge on all free elements is zero. 3 the oxidation number of combined hydrogen is. The worksheet gives students 10 compounds in which they must. The oxidation number of any pure element is 0. The oxidation number of an element in a monatomic ion equals the charge of the ion. Web 1 the oxidation number of an element is zero. The handout outlines and illustrates the rules for assigning oxidation numbers. The oxidation number of a monatomie ion equals that charge on the ion 3. The worksheet gives students 10 compounds in which they must. Amazing activity to practice the oxidation numbers. The charge on all metals of group 2 of the periodic table is +2 d. A pure element has an oxidation number of 0. Web rules for assigning oxidation numbers 1. The oxidation number of an element in a monatomic ion equals the charge of the ion. 3 the oxidation number of combined hydrogen is. The oxidation number of a monatomic ion equals the charge on the ion. The oxidation number of an element in a monatomic ion equals the charge of the ion. The worksheet gives students 10 compounds in. Web this product contains two pages. In the following questions, give the oxidation. The oxidation number of a monatomie ion equals that charge on the ion 3. The oxidation number of a monatomie ion equals that charge on the ion 3. The worksheet gives students 10 compounds in which they must. The charge on all free elements is zero. Web the oxidation number of any uncombined element is 0. Web this product contains two pages. The worksheet gives students 10 compounds in which they must. The handout outlines and illustrates the rules for assigning oxidation numbers. The worksheet gives students 10 compounds in. The charge on all metals of group 1 of the periodic table is +1 c. The worksheet gives students 10 compounds in which they must. Amazing activity to practice the oxidation numbers. The handout outlines and illustrates the rules for assigning oxidation numbers. Web this product contains two pages. The oxidation number of a monatomic ion equals the charge on the ion. Web this product contains two pages. Web use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these reactions. The worksheet gives students 10 compounds in which they must. A pure element has an oxidation number of 0. The charge on all free elements is zero. The handout outlines and illustrates the rules for assigning oxidation numbers. Identify the oxidation state of nitrogen in the following: The more electronegative clement in a binary compound is assigned the number oqual to the charge it would have if it were an ion. 3 the oxidation number of combined hydrogen is. The charge on all metals of group 2 of the periodic table is +2 d. The oxidation number of a monatomic ion equals the charge on the ion. The oxidation number of an element in a monatomic ion equals the charge of the ion. The worksheet gives students 10 compounds in which they must. Web rules for assigning oxidation numbers 1. The handout outlines and illustrates the rules for assigning oxidation numbers. It is not necessary to use. Web this product contains two pages. The oxidation number of any uncombined element is 0. The worksheet gives students 10 compounds in which they must. The more electronegative clement in a binary compound is assigned the number oqual to the charge it would have if it were an ion. Web the oxidation number of any uncombined element is 0. Web 1 the oxidation number of an element is zero. The oxidation number of a monatomie ion equals that charge on the ion 3. Web this product contains two pages. Web use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these reactions. A pure element has an oxidation number of 0. The oxidation number of a monatomic ion equals the charge on the ion.Assigning Oxidation Numbers Worksheet Answer Key Promotiontablecovers
41 Oxidation Number Worksheet Answer Key Worksheet Master
️Determining Oxidation Numbers Worksheet Answers Free Download Gmbar.co
Worksheet Oxidation Numbers Answer Key
Solved Name Date Class Redox Worksheet 1 Assigning
Oxidation Reduction Worksheet Answers
Charting Oxidation Number Worksheet Answers
41 Oxidation Number Worksheet Answer Key Worksheet Master
Oxidation Reduction Worksheet Answers
Worksheet Oxidation Numbers
Web Identify The Oxidation State Of Each Element In The Following:
2 For A Monatomic Ion, The Oxidation Number Is The Charge On The Ion.
The Charge On All Metals Of Group 1 Of The Periodic Table Is +1 C.
The Worksheet Gives Students 10 Compounds In.
Related Post: