Percent Abundance Worksheet
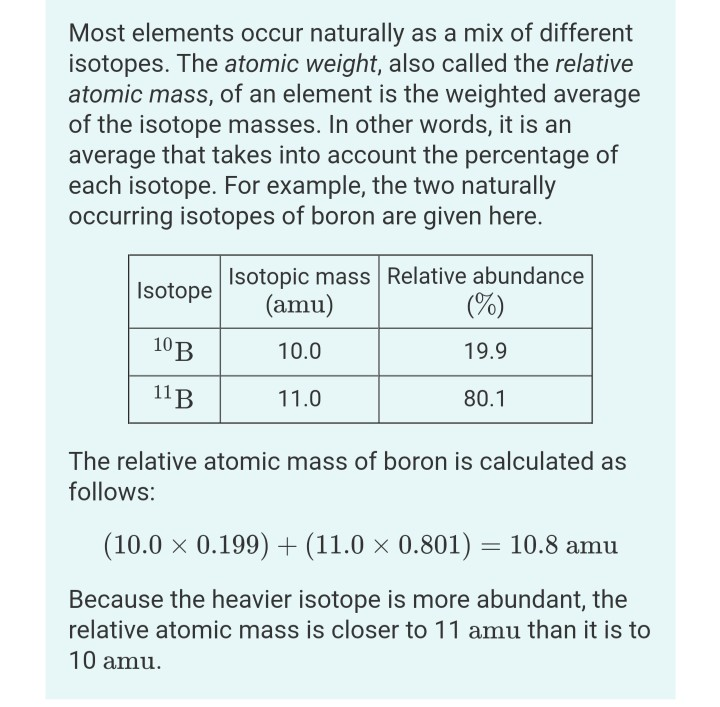
Percent Abundance Worksheet - Boost your grades with free daily practice questions. The relative abundance and atomic mass of each isotope is. Web use the following formula: The element germanium has five naturally occurring isotopes. Abundance is defined as the amount of isotope contained in its parent element. Web our percentage worksheets include finding percentage of a number, calculating percentage increase, decrease, changing decimals to and from percents,. An element has the following percent abundances. × % 2 ) +. Mass for carbon can be calculated. Web find percent abundance lesson plans and teaching resources. We're sorry, but there were no search results for percent abundance. Web use the following formula: Quickly find that inspire student learning. Web find percent abundance lesson plans and teaching resources. The element germanium has five naturally occurring isotopes. Web up to 24% cash back 1 (. Using the atomic mass on the periodic table for an element with two isotopes,. Web our percentage worksheets include finding percentage of a number, calculating percentage increase, decrease, changing decimals to and from percents,. Calculate the average atomic mass and use the periodic table to identify the element. 24mg (78.70%), 25mg (10.13%),. We're sorry, but there were no search results for percent abundance. 24mg (78.70%), 25mg (10.13%), and 26mg (11.7%). An element has the following percent abundances. Web this is a simple worksheet to show how the average mass is calculated by several methods. × % 2 ) +. Web math explained in easy language, plus puzzles, games, quizzes, videos and worksheets. 24mg (78.70%), 25mg (10.13%), and 26mg (11.7%). Percent abundance lesson plans & worksheets reviewed by teachers Web our percentage worksheets include finding percentage of a number, calculating percentage increase, decrease, changing decimals to and from percents,. Mass for carbon can be calculated. Web our percentage worksheets include finding percentage of a number, calculating percentage increase, decrease, changing decimals to and from percents,. Mass for carbon can be calculated. Web use the following formula: X denotes its relative abundance. We're sorry, but there were no search results for percent abundance. Mass for carbon can be calculated. The first is by multiplying the number of atoms my their mass and then adding. 24mg (78.70%), 25mg (10.13%), and 26mg (11.7%). We're sorry, but there were no search results for percent abundance. Web this is a simple worksheet to show how the average mass is calculated by several methods. Web according to chemistry principles, isotopes have same atomic number but different mass number. Web math explained in easy language, plus puzzles, games, quizzes, videos and worksheets. An element has the following percent abundances. Web our percentage worksheets include finding percentage of a number, calculating percentage increase, decrease, changing decimals to and from percents,. Abundance is defined as the amount. An element has the following percent abundances. Web math explained in easy language, plus puzzles, games, quizzes, videos and worksheets. X denotes its relative abundance. Web up to $3 cash back using both the relative abundance and the masses for each of these two isotopes. The relative abundance and atomic mass of each isotope is. Calculate the average atomic mass and use the periodic table to identify the element. 24mg (78.70%), 25mg (10.13%), and 26mg (11.7%). The first is by multiplying the number of atoms my their mass and then adding. Mass for carbon can be calculated. Web according to chemistry principles, isotopes have same atomic number but different mass number. Abundance is defined as the amount of isotope contained in its parent element. Web our percentage worksheets include finding percentage of a number, calculating percentage increase, decrease, changing decimals to and from percents,. Web up to 24% cash back 1 (. Web the percent abundance of these isotopes is as follows: X denotes its relative abundance. Web math explained in easy language, plus puzzles, games, quizzes, videos and worksheets. Web determine the average atomic mass from the natural isotopic distribution of the atoms of an element. The first is by multiplying the number of atoms my their mass and then adding. Boost your grades with free daily practice questions. Web find percent abundance lesson plans and teaching resources. Using the atomic mass on the periodic table for an element with two isotopes,. Web this article will show you the percent abundance formula, how to calculate it step by step and give you examples on how the formula can be utilized. We're sorry, but there were no search results for percent abundance. Web pdf, 301.43 kb. × % 2 ) +. Web up to 24% cash back 1 (. Abundance is defined as the amount of isotope contained in its parent element. The element germanium has five naturally occurring isotopes. Using the equation below, the atomic. Web our percentage worksheets include finding percentage of a number, calculating percentage increase, decrease, changing decimals to and from percents,. Percent abundance lesson plans & worksheets reviewed by teachers The relative abundance and atomic mass of each isotope is. The average atomic mass of the three isotopes is 24.3050 amu. X denotes its relative abundance. 24mg (78.70%), 25mg (10.13%), and 26mg (11.7%). Web up to 24% cash back 1 (. The mass of the second isotope is denoted. Percent abundance lesson plans & worksheets reviewed by teachers Quickly find that inspire student learning. Web the percent abundance of these isotopes is as follows: Web up to $3 cash back using both the relative abundance and the masses for each of these two isotopes. The first is by multiplying the number of atoms my their mass and then adding. Boost your grades with free daily practice questions. Web find percent abundance lesson plans and teaching resources. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes and percentage. X denotes its relative abundance. × % 2 ) +. Web this is a simple worksheet to show how the average mass is calculated by several methods. Web according to chemistry principles, isotopes have same atomic number but different mass number. The relative abundance and atomic mass of each isotope is. Abundance is defined as the amount of isotope contained in its parent element.Isotope2 percent abundance YouTube
ShowMe percent abundance
How To Calculate Percent Abundance Formula
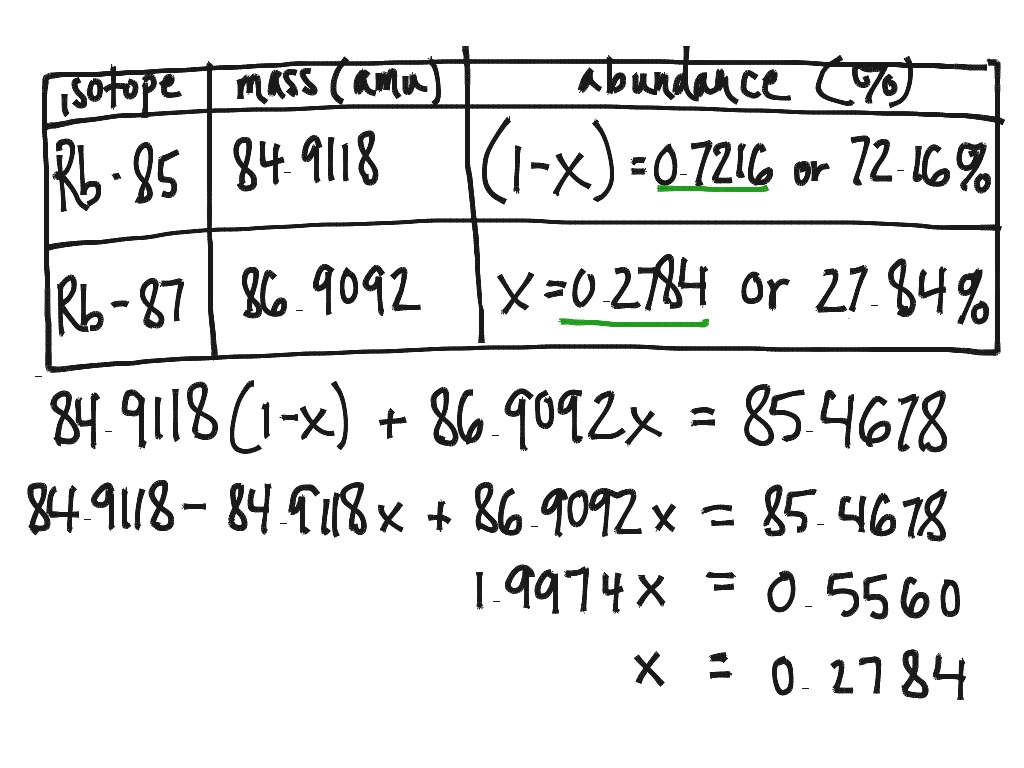
Average Atomic Mass and Percent Abundance Worksheet 2 and KEY Isotope
How To Calculate Percent Abundance In Chemistry
Calculating Percent Abundance Of Isotopes Worksheet worksheet
How To Calculate Percent Abundance Formula
How To Calculate Percent Abundance Of Each Isotope
How To Calculate Relative Abundance
Calculating Percentage abundance of each isotope YouTube
Using The Equation Below, The Atomic.
Using The Atomic Mass On The Periodic Table For An Element With Two Isotopes,.
24Mg (78.70%), 25Mg (10.13%), And 26Mg (11.7%).
The Element Germanium Has Five Naturally Occurring Isotopes.
Related Post: