Percent Composition And Empirical Formula Worksheet
Percent Composition And Empirical Formula Worksheet - The first worksheet gives your students practice with finding the percent composition of a compound from a given formula, finding percent. Web determine the molecular formula for each compound whose percentage composition is shown below. Web if the percent composition is as follows, what is the empirical and molecular formula of serine? Percentage composition and empirical formulas. 84.9% hg and the remainder cl, with a molecular weight of 472.2 g/mol. Empirical formulas work each of the following problems. Web percent composition and molecular formula worksheet solutions 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0%. C = 69.40 % h= 5.825 % o = 13.21 % n= 11.57 % a component of protein called serine has an approximate molar mass of 100 g/mole. A compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per. What is the percent composition of calcium. S = _____ % o = _____ % 2. Web as you work through this unit, you should be able to: A compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per. This unit is meant to cover the basics of. Web what is the empirical formula? Web an empirical formula is the lowest common ratio between the elements in a compound. Web a compound with an empirical formula of cfbro and a molar mass of 254 grams per mole. Web there are two kinds of percents here: Web if the percent composition is as follows, what is the empirical and molecular formula of serine? 84.9% hg. Web there are two kinds of percents here: The mass fraction and the mole fraction. The first worksheet gives your students practice with finding the percent composition of a compound from a given formula, finding percent. Before viewing an episode, download and print the note. % composition and empirical formulas part 1: Web if the percent composition is as follows, what is the empirical and molecular formula of serine? The molecular formula is an actual, real formula. Web calculate the empirical formula of the compound whose percent composition is: % composition name _________________ calculate the percent composition of the following compounds. Web this is a package of two worksheets. Empirical formulas work each of the following problems. 84.9% hg and the remainder cl, with a molecular weight of 472.2 g/mol. The mass fraction and the mole fraction. Percentage composition and empirical formulas. % composition and empirical formulas part 1: Web the second worksheet asks students to predict empirical and molecular formulas from given inform subjects: So what the percentage is depends on what kind of percent you're talking about. Before viewing an episode, download and print the note. Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of stoichiometry! % composition. In order to calculate a molecular. Determine the empirical formula of a compound containing 63.50 % silver, 8.25 %. Web the second worksheet asks students to predict empirical and molecular formulas from given inform subjects: Percent composition % is based on the mass of an item that is grams to the rest of you! Web a compound with an empirical. Web the second worksheet asks students to predict empirical and molecular formulas from given inform subjects: Before viewing an episode, download and print the note. This unit is meant to cover the basics of. Web empirical formulas, and molecular formulas 1. Web percent composition and molecular formula worksheet solutions 1) what’s the empirical formula of a molecule containing 65.5% carbon,. C = 69.40 % h= 5.825 % o = 13.21 % n= 11.57 % a component of protein called serine has an approximate molar mass of 100 g/mole. Web what is the empirical formula? Web an empirical formula is the lowest common ratio between the elements in a compound. So what the percentage is depends on what kind of percent. 84.9% hg and the remainder cl, with a molecular weight of 472.2 g/mol. Web determine the molecular formula for each compound whose percentage composition is shown below. % composition and empirical formulas part 1: Web as you work through this unit, you should be able to: What is the percent composition of so 2? In order to calculate a molecular. The molecular formula is an actual, real formula. Web percent composition and empirical formula. What is the percent composition of so 2? Web determine the molecular formula for each compound whose percentage composition is shown below. C = 69.40 % h= 5.825 % o = 13.21 % n= 11.57 % a component of protein called serine has an approximate molar mass of 100 g/mole. Web what is the empirical formula? The mass fraction and the mole fraction. A compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per. Web empirical formulas, and molecular formulas 1. What is the percent composition of calcium. Web this is a package of two worksheets. Percentage composition and empirical formulas. Calculate the percent composition of a substance from its chemical formula or experimental data. Empirical formulas work each of the following problems. 57.48 % sodium (na), 39.99 % oxygen (o), and 2.52 % hydrogen (h) 57.48 % of na becomes. Web percent composition and molecular formula worksheet solutions 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0%. Percent composition % is based on the mass of an item that is grams to the rest of you! Web calculate the empirical formula of the compound whose percent composition is: The first worksheet gives your students practice with finding the percent composition of a compound from a given formula, finding percent. Percentage composition and empirical formulas. Calculate the percent composition of a substance from its chemical formula or experimental data. Web this is a package of two worksheets. In order to calculate a molecular. The molecular formula is an actual, real formula. Web calculate the empirical formula of the compound whose percent composition is: Web percent composition and empirical formula. Web an empirical formula is the lowest common ratio between the elements in a compound. Percent composition % is based on the mass of an item that is grams to the rest of you! Before viewing an episode, download and print the note. Web empirical formulas, and molecular formulas 1. Web the second worksheet asks students to predict empirical and molecular formulas from given inform subjects: This unit is meant to cover the basics of. What is the percent composition of calcium. 84.9% hg and the remainder cl, with a molecular weight of 472.2 g/mol. S = _____ % o = _____ % 2.Percent Composition And Empirical Formula Quiz Shop
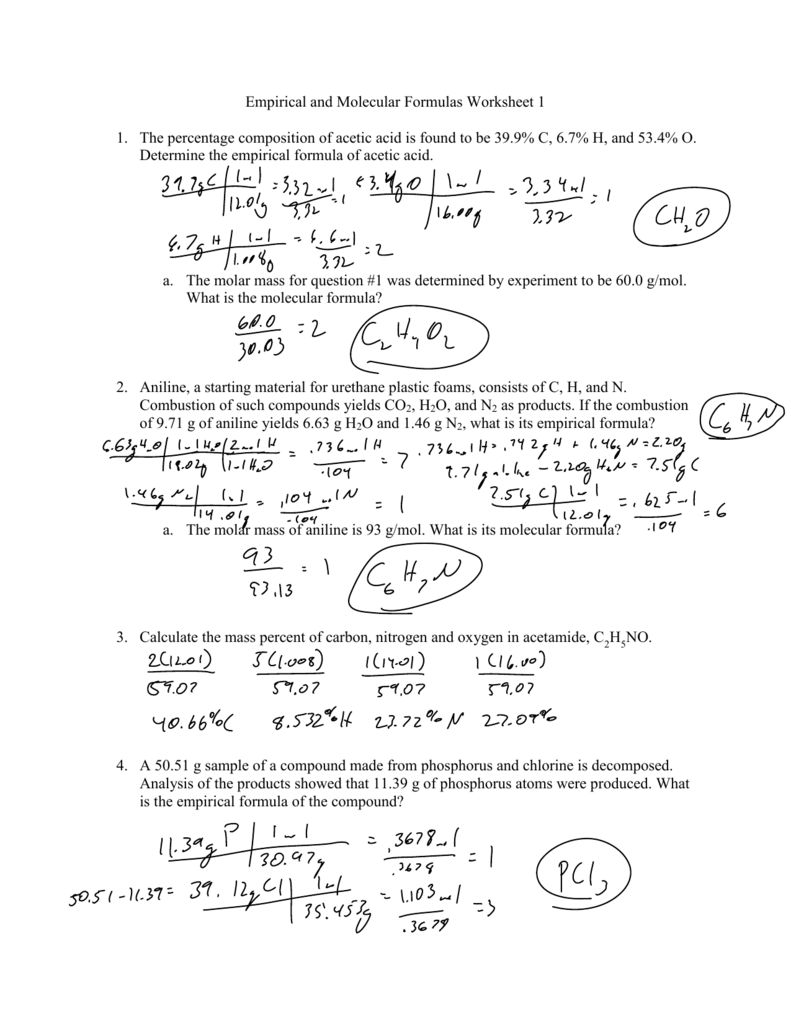
Empirical and Molecular Formulas Worksheet 1 1. The percentage
Percent Composition And Empirical Formula Worksheet Ivuyteq
Percent Composition, Empirical Formulas, and Molecular Formulas
empirical formula worksheet 1
comp, Emp & molec Formula MS MCLARTY'S CLASSES
Empirical Formula Worksheet Answers With Work Organicfer
Quiz & Worksheet How to Calculate Percent Composition and Determine
8 Best Images of Percent Composition Worksheet Answer Key Percent
comp, Emp & molec Formula MS MCLARTY'S CLASSES
% Composition And Empirical Formulas Part 1:
Web Determine The Molecular Formula For Each Compound Whose Percentage Composition Is Shown Below.
Web Percent Composition And Molecular Formula Worksheet Solutions 1) What’s The Empirical Formula Of A Molecule Containing 65.5% Carbon, 5.5% Hydrogen, And 29.0%.
Web What Is The Empirical Formula?
Related Post: